Figures & data
Figure 1. MiRNA-324-5p is highly expressed in GC
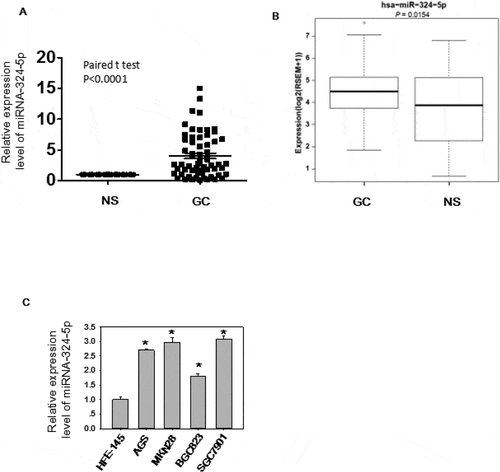
Figure 2. MiRNA-324-5p has an oncogenic effect in GC
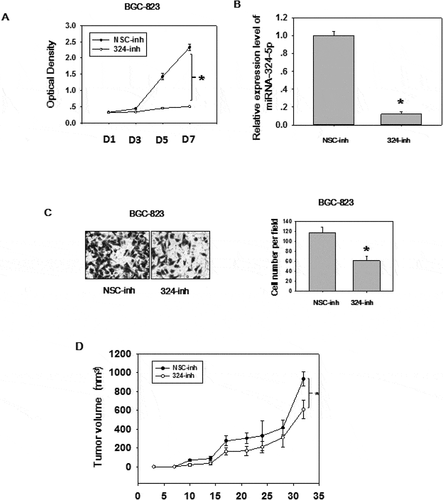
Figure 3. MiRNA-324-5p activates Wnt/β-catenin signaling pathway and EMT. (a)MiRNA-324-5p activated Topflash luciferase activity. BGC-823 cells were co-transfected with NSC-inh or miRNA-324-5p inhibitors, Topflash/Fopflash plasmids and pTK-renilla. *p < 0.05. (b) MiRNA-324-5p activated Topflash luciferase activity. HFE-145 cells were co-transfected with NSC-mim or miRNA-324-5p mimics, Topflash/Fopflash plasmids and pTK-renilla. *p < 0.05. (c) Suppression of miRNA-324-5p inhibited the translocation of β-catenin into the nucleus. Forty-eight hours after miRNA-324-5p inhibitors transfection, BGC-823 cells were lysed and immunoblotted with β-catenin antibody in cytoplasmic and nuclear portion, respectively. (d) Inhibition of miRNA-324-5p blocked the translocation of β-catenin into the nucleus. β-catenin localization was detected by confocal immunofluorescence 48 h after transfection of miRNA-324-5p inhibitors in BGC-823 cells. (e) MiRNA-324-5p activated Wnt/β-catenin downstream gene expression. Cell lysates were used to blot Wnt downstream gene antibody 48 h after transfection of miRNA-324-5p inhibitors or mimics in BGC-823 and HFE-145 cells, respectively. (f) MiRNA-324-5p induced EMT. Cell lysates were used to blot EMT markers 48 h after transfection of miRNA-324-5p inhibitors or mimics in BGC-823 and HFE-145 cells, respectively. Each experiment was repeated three times
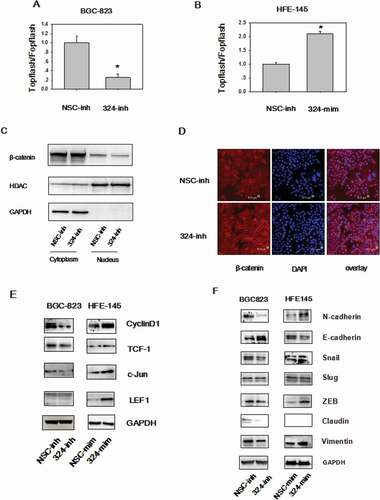
Figure 4. SUFU is the direct target of miRNA-324-5p. (a) The expression level of SUFU was up-regulated by miRNA-324-5p inhibitors in BGC-823 and down-regulated by miRNA-324-5p mimics in HFE-145 cells. (b) The predicted binding sites of miRNA-324-5p on the 3’UTR region of SUFU and their corresponding mutant sites in luciferase reporters are shown. (c) HFE-145 and BGC-823 cells were co-transfected with miRNA-324-5p mimics/inhibitors and SUFU 3’UTR WT (wild-type)/Mutant reporter plasmids. SUFU 3’ UTR dependent luciferase activities were reduced or increased by miRNA-324-5p mimics or inhibitors, respectively,*p < 0.05. (d) SUFU expression level in 60 paired GC tissues was determined by qRT-PCR and SUFU was down expressed in GC. (e)RNA-seq analysis of SUFU mRNA levels in 368 GCs and 35 normal tissues queried from TCGA showed that SUFU was down-regulated in GC, *p < 0.05. (f) Spearman’s correlation analysis showed that there was a negative correlation between SUFU and miRNA-324-5p expression in 60 tested tissues, *p < 0.001. (g) Expression correlation analysis was performed between miRNA-324-5p and SUFU in GCs from TCGA database (n = 368), r = −0.108, p = 0.038. SUFU expression was negatively correlated with that of miRNA-324-5p
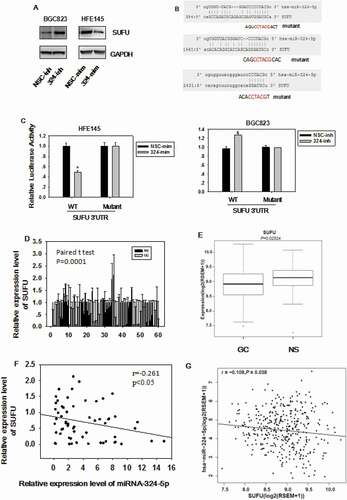
Figure 5. MiRNA-324-5p activates Wnt/β-catenin signaling pathway and EMT via SUFU. (a) Topflash luciferase activity was decreased by miRNA-324-5p inhibitors and SUFU siRNAs partially reversed this inhibitory effect. BGC-823 cells were transfected with miRNA-324-inh or co-transfected with miRNA-324-inh and SUFU siRNAs. (b) Interference of SUFU relieved the cytoplasmic retention of β-catenin induced by miRNA-324-5p blockage. (c) β-catenin translocation to nuclei induced by miRNA-324-5p inhibition was reversed by the coupled SUFU knockdown. Confocal immunofluorescence was performed to locate the β-catenin cytoplasmic or nuclear localization 48 h after transfection. (d) Suppression of SUFU alleviated the EMT induced by miRNA-324-5p inhibition. Each experiment was repeated three times
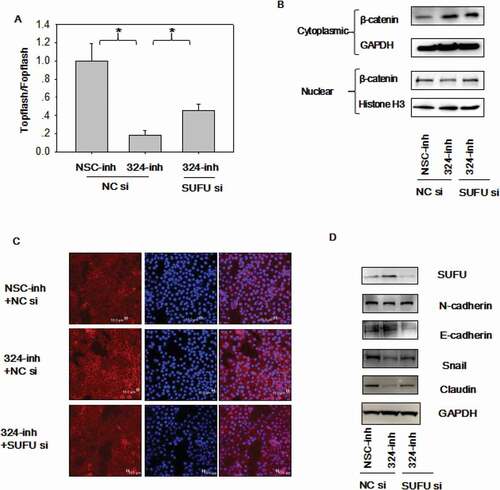
Figure 6. MiRNA-324-5p promotes cell migration and colony formation ability by activating Wnt/β-catenin signaling pathway via SUFU. (a-b)Suppression of miRNA-324-5p weaken the migration capacity of BGC-823 cells while SUFU siRNAs can alleviate this inhibition. BGC-823 cells were transfected with miRNA-324-inh or co-transfected with miRNA-324-inh and SUFU siRNAs. Forty-eight hours after transfection, scratch assay and transwell assay were conducted. (c) MiRNA-324-5p inhibitors impaired colony forming capacity in BGC-823 cells while SUFU siRNAs reversed the depressive effect. 48 h after transfection with either miRNA-324-5p inhibitors or coupled SUFU siRNAs, 200 cells/well were inoculated into a 6-well plate. Cell colonies were stained and counted 14 days after plating. Each experiment was repeated three times. *p < 0.05
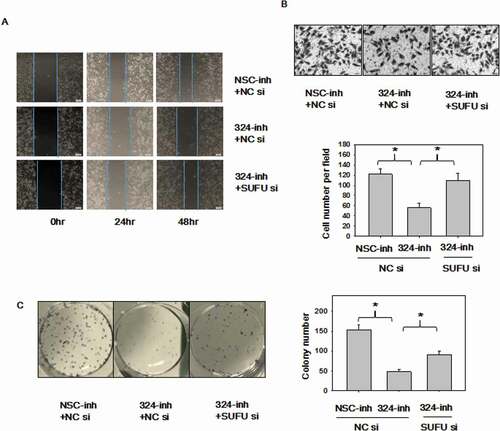
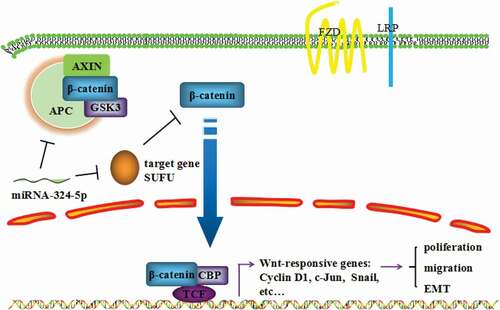
Figure 7. MiRNA-324-5p promotes cell proliferation, migration and EMT by activating Wnt/β-catenin signaling pathway via SUFU
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon request.
