Figures & data
Figure 2. MiR-17-5p increased the numbers of local EPCs and circulating EPCs and facilitated the vascular repair of aneurysm. (a) The histopathology of aneurysm tissues was examined by hematoxylin-eosin staining (magnification ×200). (b) The vWF expression in the aneurysm tissues was determined using immunofluorescence (magnification ×200). (c) The KDR expression in the aneurysm tissues was determined using immunofluorescence (magnification ×200). (d-e) The percentage of EPCs in the mononuclear cells of rat peripheral blood was detected using flow cytometry (^^^P < 0.001, vs. Sham; ***P < 0.001, vs. Model+AgomiR-NC). (EPCs: endothelial progenitor cells, NC: negative control)
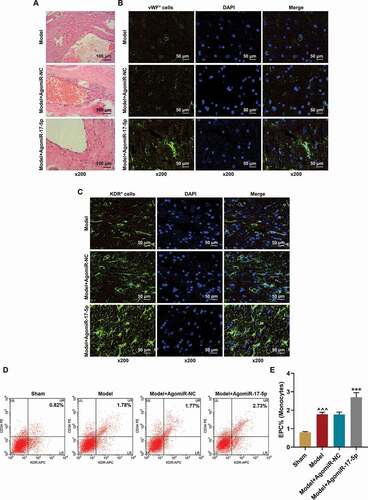
Figure 3. EPCs from healthy rats were successfully isolated and cultured. (a) The morphology of the isolated EPCs. (b-c) The percentages of KDR+, CD34+, and double positive cells in the isolated EPCs were determined using flow cytometry. (EPCs: endothelial progenitor cells)
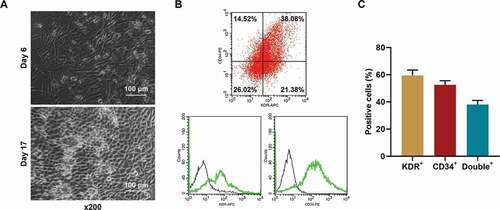
Figure 4. MiR-17-5p regulated the viability, migration, and tube formation of EPCs. (a) The transfection efficiency of miR-17-5p was evaluated using qPCR, with U6 as an internal control. (b) The viability of EPCs transfected with miR-17-5p mimic and inhibitor was detected by MTT assays. (c-e) The migration ability of EPCs transfected with miR-17-5p mimic and inhibitor was detected by wound healing assays (magnification ×100). (d-f) The tube formation ability of EPCs transfected with miR-17-5p mimic and inhibitor was detected by tube formation assays (magnification ×100). (***P < 0.001, vs. MC; ^P < 0.05, ^^^P < 0.001, vs. IC) (EPCs: endothelial progenitor cells, M: miR-17-5p mimic, I: miR-17-5p inhibitor, MC: mimic control, IC; inhibitor control)
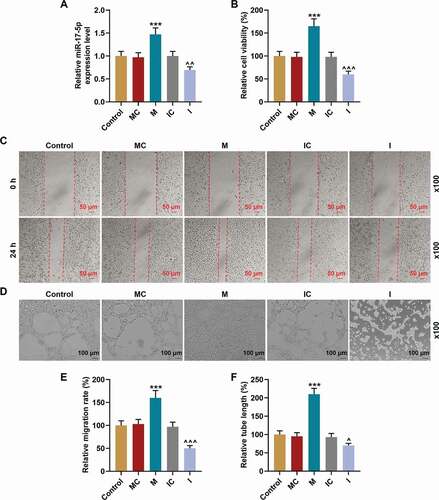
Figure 5. MiR-17-5p regulated the expression of VEGFA and the activation of PTEN-mediated PI3K/AKT pathway. (a) The secretion of VEGFA in EPCs transfected with miR-17-5p mimic and inhibitor was detected by ELISA. (b-c) The expressions of PTEN, p-PI3K, PI3K, p-AKT, AKT, and VEGFA in EPCs transfected with miR-17-5p mimic and inhibitor were detected by Western blot. β-actin was used as an internal control. (d-e) The ratios of p-PI3K to PI3K (d) and p-AKT to AKT (e) were calculated based on the data of Western blot. (***P < 0.001, vs. MC; ^^^P < 0.001, vs. IC) (EPCs: endothelial progenitor cells, M: miR-17-5p mimic, I: miR-17-5p inhibitor, MC: mimic control, IC; inhibitor control)
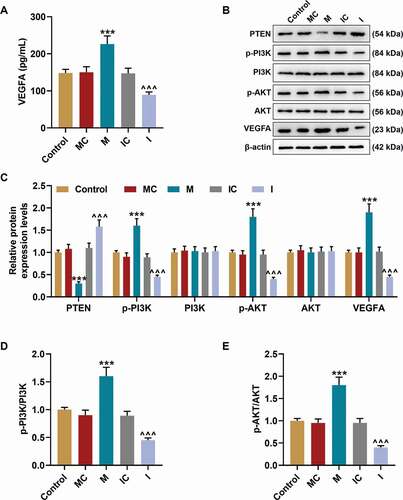
Figure 6. PTEN was targeted by miR-17-5p and overturned the effects of miR-17-5p mimic on the expression of PTEN and the viability of ECPs. (a) The binding sites between PTEN and miR-17-5p were predicted by Targetscan7.2. (b) The targeted relationship between PTEN and miR-17-5p was verified by dual-luciferase reporter assay. (c-d) The expression of PTEN in EPCs after overexpressing miR-17-5p and PTEN was detected by Western blot. β-actin was used as an internal control. (e) The expression of PTEN in EPCs after overexpressing miR-17-5p and PTEN was detected by qPCR. β-actin was used as an internal control. (f) The viability of EPCs after overexpressing miR-17-5p and PTEN was detected by MTT assay. (+++P < 0.001, vs. MC; ***P < 0.001, vs. NC; ^^^P < 0.001, vs. Control; ###P < 0.001, vs. PTEN; &&&P < 0.001, vs. M+ NC) (EPCs: endothelial progenitor cells, M: miR-17-5p mimic, MC: mimic control, NC; negative control)
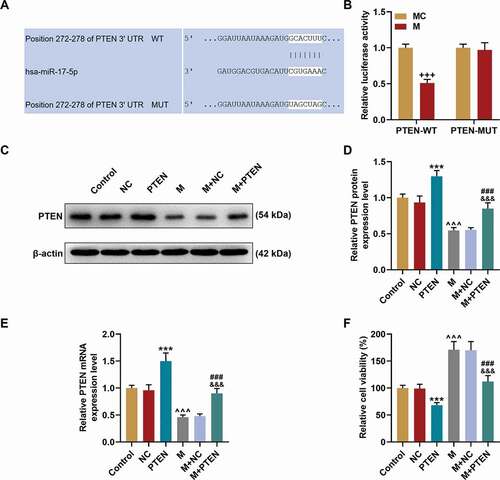
Figure 7. PTEN overexpression overturned the effects of miR-17-5p mimic on the migration and tube formation of EPCs. (a, c) The migration ability of EPCs after overexpressing miR-17-5p and PTEN was detected by wound healing assay (magnification ×100). (B. D) The tube formation ability of EPCs after overexpressing miR-17-5p and PTEN was detected by tube formation assay (magnification ×100). (***P < 0.001, vs. NC; ^^^P < 0.001, vs. Control; ###P < 0.001, vs. PTEN; &&&P < 0.001, vs. M+ NC) (EPCs: endothelial progenitor cells, M: miR-17-5p mimic, NC; negative control)
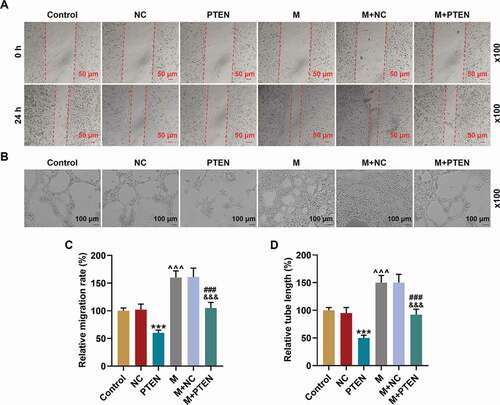
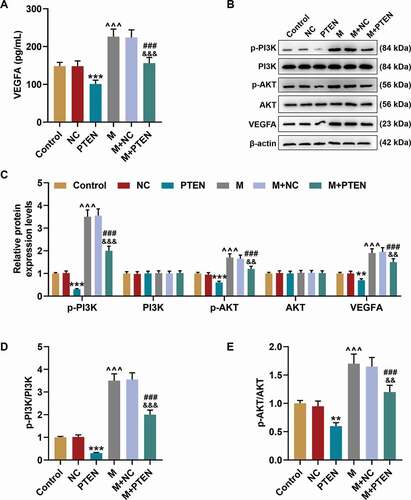
Figure 8. PTEN overexpression overturned the effects of miR-17-5p mimic on the expression of VEGFA and the activation of PTEN-mediated PI3K/AKT pathway. (a) The secretion of VEGFA in EPCs after overexpressing miR-17-5p and PTEN was detected by ELISA. (b-c) The expressions of p-PI3K, PI3K, p-AKT, AKT, and VEGFA in EPCs after overexpressing miR-17-5p and PTEN were detected by Western blot. β-actin was used as an internal control. (d-e) The ratios of p-PI3K to PI3K (d) and p-AKT to AKT (e) were calculated based on the data of Western blot. (***P < 0.001, vs. NC; ^^^P < 0.001, vs. Control; ###P < 0.001, vs. PTEN; &&&P < 0.001, vs. M+ NC) (EPCs: endothelial progenitor cells, M: miR-17-5p mimic, NC; negative control)