Figures & data
Table 1. Table listing neuronal or spermatogenic defects observed in knock-out mice models for RBPs involved in splicing regulation
Figure 1. Common regulation by PTBP2 of Cdc42 splicing in both brain and testes. The tissue-specific splicing factor PTBP2 binds to the Cdc42 pre-mRNA, hence regulating usage of the two alternative last exons E6 or E7. This splicing event regulates axonogenesis in neurons and interaction between germ cells and nursing Sertoli cells in testis
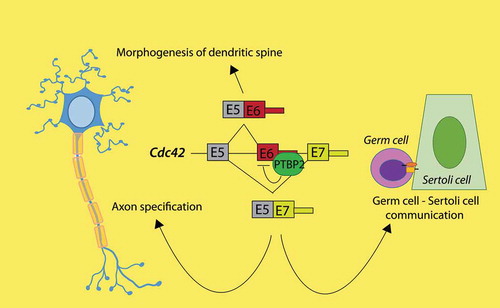
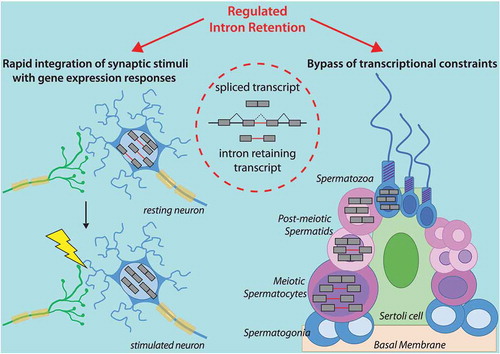
Figure 2. Regulated intron retention compensates physiological transcriptional constraints in neurons and male germ cells. Schematic representation of an intron retention event. The left panel illustrates the accumulation of intron-retaining transcript in resting neurons (upper) and prompt splicing of these introns in stimulated neurons (lower). This mechanisms ensures faster translation of synaptic stimuli in gene expression responses than de novo transcription alone. The right panel illustrates the accumulation of intron-retaining transcripts in meiotic cells which are later spliced in post-meiotic cells during the transcriptionally silent phases of spermatogenesis