Figures & data
Figure 1. The interplay between DDR and cellular metabolism. The players of the different DDR pathways can differentially regulate the energetic metabolism. ATM was shown to upregulate the expression of glucose transporters and to promote the activity of G6PD and the PPP, leading also to the synthesis of nucleotide and production of glutathione to counteract ROS. ATM can also promote mitochondrial metabolism by increasing the activity of complex I. p53 instead, can inhibit glucose uptake and glycolysis, favouring the activity of PPP. Furthermore, p53 can induce the synthesis of nucleotide and glutathione and increase mitochondrial activity promoting the assembly of complex IV. DNA-PK is able to induce lipogenesis and FAS whilst downregulating the number of mitochondria during ageing. Amongst HR genes, BRCA1 can inhibit the activity of glycolysis to favour mitochondrial metabolism through the TCA cycle; whilst in the context of MMR, MLH1 is important for the regulation of complex I activity and the inhibition of ROS production. Loss of NER activity (ERCC1-/-) leads to inhibition of glycolysis favouring the re-routing of glucose towards PPP. Finally, in the context of BER, loss of XRCC1 is associated with increased expression of AA-transporters and upregulation of serine biosynthesis; whilst increased PARP activity leads to compromising of glycolysis. GLUT – glucose transporters; AA – amino acids transporters; G6PD – glucose-6-phosphate dehydrogenase; PPP – pentose phosphate pathway; ROS – reactive oxygen species; TCA cycle – tricarboxylic acid cycle; ETC – electron transport chain; DDR – DNA damage response; HR – homologous recombination; MMR – mismatch repair; NER – nucleotide excision repair; BER – base excision repair
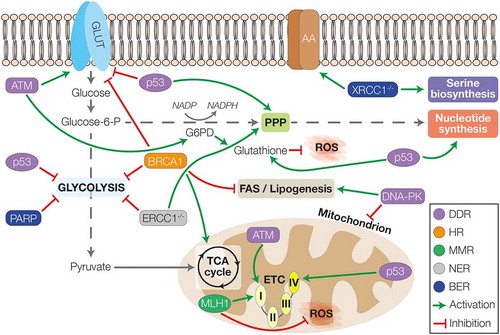
Table 1. Therapeutic agents targeting metabolism for cancer treatment; either approved, in clinical trials, or undergoing preclinical research
