Figures & data
Figure 1. Knock down of MAGT1 inhibits proliferation and induces S-phase arrest and apoptosis in HeLa cells
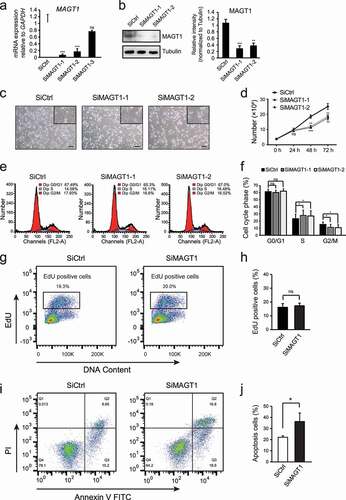
Figure 2. MAGT1 regulates several cell cycle related gene expressions
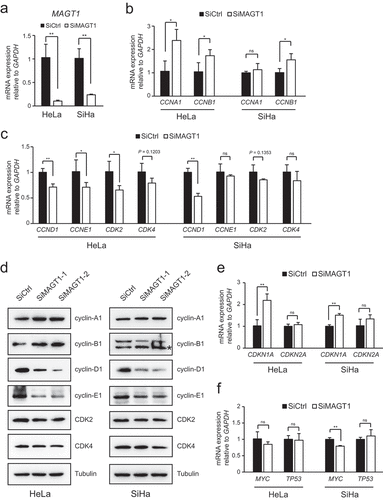
Figure 3. MAGT1 regulates a large batch of target gene expression
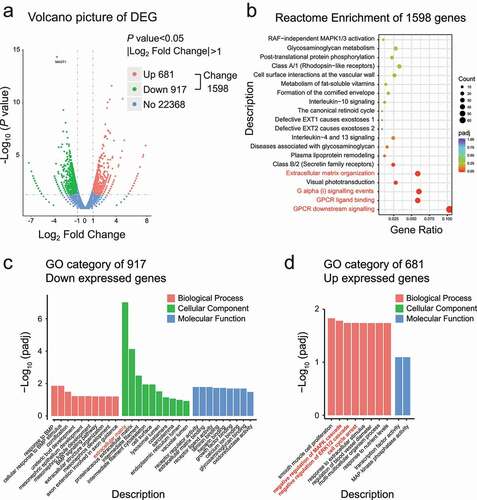
Figure 4. Downregulation of MAGT1 causes the reduction of ERK/p38 MAPK signaling pathways
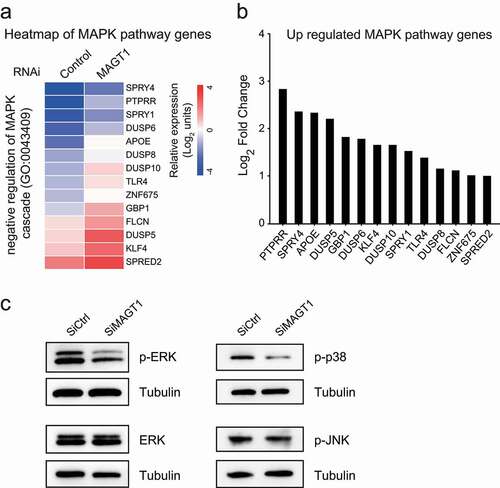
Figure 5. MAGT1 is required for E6/E7 proliferation regulation and G1/S transition
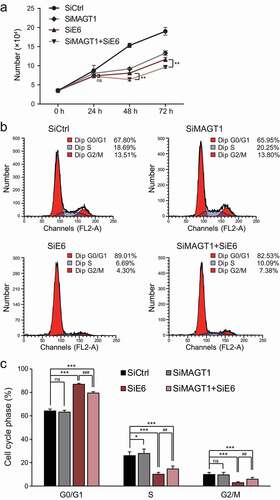
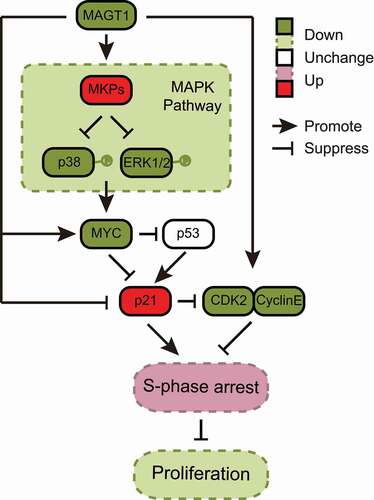
Figure 6. Molecular mechanism of MAGT1 in HeLa cell proliferation
Data availability statement
The GEO accession number for the RNA-seq data is GSE166084. The data that support the findings of this study are available from the corresponding author upon reasonable request. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE166084
