Figures & data
Figure 1. GPR30 mediates 17β-estradiol-induced cell proliferation of gMECs. Cell counting assay (a), BrdU incorporation assay (b), and CCK-8 assay (c) were performed to detect the effect of 17β-estradiol (E2, 0.01, 0.1, and 1 μM) for 5 d on cell proliferation. Determination of GPR30 expression by immunofluorescence (d) and Western blot (e). Cell counting assay (f), BrdU incorporation assay (g), and CCK-8 assay (h) were utilized to detect the effect of 17β-estradiol (0.1 μM), G1 (0.1 μM), and 17β-estradiol (0.1 μM) + G15 (1 μM) for 5 d on cell proliferation, respectively. β-Actin serves as a loading control. P3, P6, P9, the 3, 6, and 9 generation of gMECs. Scale bar = 50 µm. nsp > 0.05, *p < 0.05, **p < 0.01.
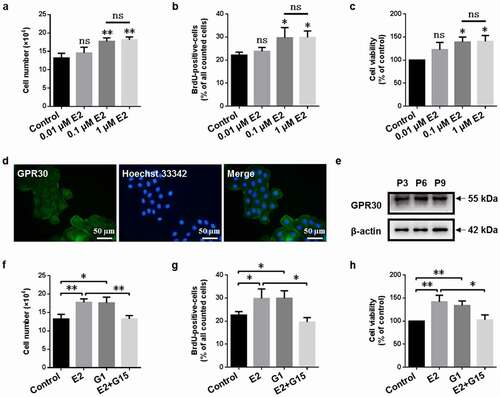
Figure 2. GPR30 silencing suppresses cell proliferation of gMECs. (a) Western blot was used to determine the protein level of GPR30 in gMECs. Cell counting assay (b), BrdU incorporation assay (c), and CCK-8 assay (d) were performed to assess cell proliferation of gMECs with GPR30 silencing. β-Actin serves as a loading control. *p < 0.05, **p < 0.01, ***p < 0.001.
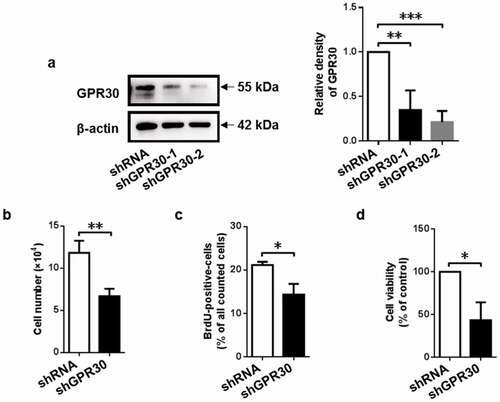
Figure 3. GPR30 silencing arrests cell cycle progression of gMECs. (a) Flow cytometry was used to analyze the distribution of cell cycle of gMECs with GPR30 silencing. (b) The expression of cell cycle checkpoint regulators of gMECs with GPR30 silencing was detected by Western blot. β-Actin serves as a loading control. nsp > 0.05, *p < 0.05.
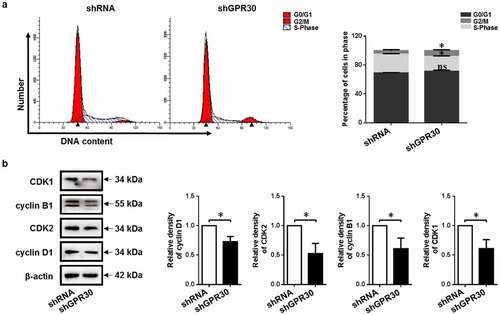
Figure 4. 17β-Estradiol binding to GPR30 activates MEK/ERK and PI3K/AKT signaling pathway. (a, c) Western blot was performed to detect the protein level of p-ERK1/2 and p-AKT of gMECs cultured with 17β-estradiol (0.1 μM), G1 (0.1 μM), and 17β-estradiol (0.1 μM) + G15 (1 μM) for 24 h, respectively. (b, d) The protein level of p-ERK1/2 and p-AKT of gMECs with GPR30 silencing were detected by Western blot, respectively. β-Actin serves as a loading control. *p < 0.05, **p < 0.01.
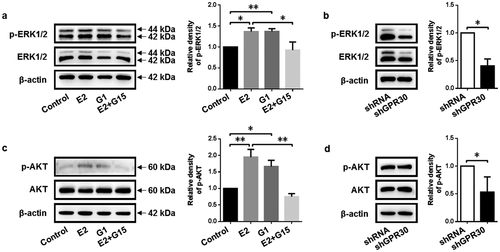
Figure 5. Inhibition of MEK/ERK and PI3K/AKT signaling pathway arrests cell cycle progression of gMECs. (a, b) The protein expression of cell cycle checkpoint regulators was detected by Western blot after treatment with MEK inhibitor U0126 (10 μM) and PI3K inhibitor LY294002 (10 μM) in the presence of 17β-estradiol (0.1 μM) and G1 (0.1 μM) for 48 h, respectively. β-Actin serves as a loading control. *p < 0.05, **p < 0.01, ***p < 0.001.
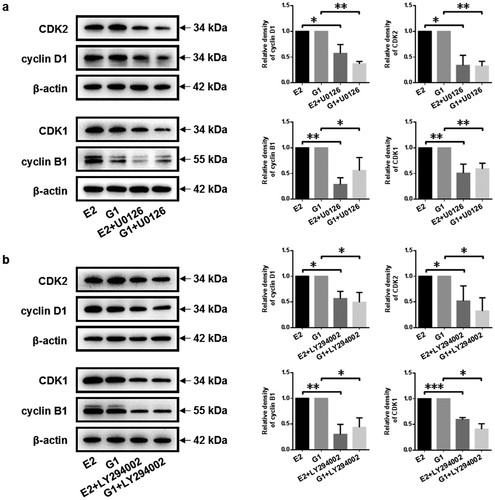
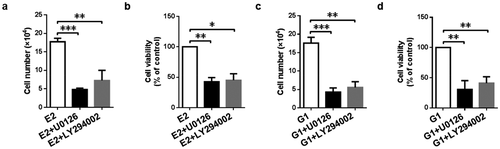
Figure 6. 17β-Estradiol-induced and GPR30-mediated cell proliferation via MEK/ERK and PI3K/AKT signaling pathway. Cell counting assay (a, c) and CCK-8 assay (b, d) were implemented to evaluate cell proliferation after treatment with MEK inhibitor U0126 (10 μM) and PI3K inhibitor LY294002 (10 μM) in the presence of 17β-estradiol (0.1 μM) and G1 (0.1 μM) for 5 d, respectively. *p < 0.05, **p < 0.01, ***p < 0.001.