Figures & data
Figure 1. Loss of p18 stimulates follicular cell proliferation and induces bilateral papillary thyroid tumors. (a) Representative gross appearance and HE staining (inset) of the thyroid tumors from a 9-month-old p18−/− female mouse. Note the thyroid tumors in both sides of the esophagus (Eso.), Th., Tu., thyroid tumor. (b) Representative HE staining of p18−/− thyroid tumors. Note typical pathological PTC features including tumor cells containing enlarged, overlapping nuclei (blue arrows), cells with Orphan Annie nuclei (black arrows), and cells with nuclear grooves and nuclear membrane irregularities (yellow arrows). (c) Body weight analysis of p18−/− mice with or without thyroid tumors. Data represent the mean ± SD of five mice in 2–4 month females without thyroid tumor group, four mice in 2–4 month females with thyroid tumor group, six mice in 4–12 month females without thyroid tumor group, and four mice in 4–12 month females with thyroid tumor group. * p < 0.05 between the group without thyroid tumor and the group with thyroid tumor. (d) IHC analysis of thyroid tumors developed in p18−/− mice. Calcitonin-positive cells are indicated by arrows. Inset shows the representative IHC analysis of tumor-free thyroid. Note, the majority of tumor cells are negative for calcitonin, other than a few sporadically located calcitonin-positive cells. (e) IHC analysis of tumor-free thyroids from WT and p18−/− mice at the age of 2–4 months of age. The percentages of Ki67-positive cells were calculated from cells situated in clear duct/gland structures. Results represent the mean ± SD of three animals per group.
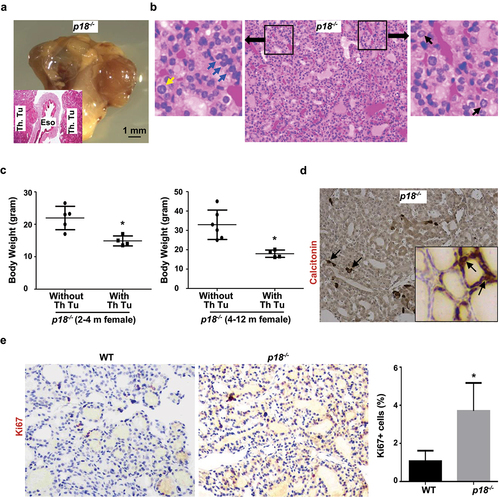
Figure 2. Heterozygous germline deletion of Brca1 in p18 null mice promotes EMT and enhances metastasis of thyroid tumors. (a) HE staining analysis of thyroid tumors developed in mutant mice. Note relative to p18−/− thyroid tumor cells, p18−/−;Brca1+/- cells are highly heterogenous and poorly differentiated. Tumor a and b show two thyroid tumors derived from two individual p18−/−;Brca1+/- mice. Picture in the most right shows lung metastasis from thyroid tumor b. (b, c) IHC analysis of thyroid tumors developed in mutant mice. Note relative to p18−/− tumor cells, more p18−/−;Brca1+/- cells expresses strong VIM and TWIST, but none or weak TTF1, CK19, Galectin-3, and HBME1. (d) IHC analysis of thyroid tumors with an antibody against Ki67. The percentages of Ki67-positive cells were calculated and plotted. Results represent the mean ± SD of three tumors per group.
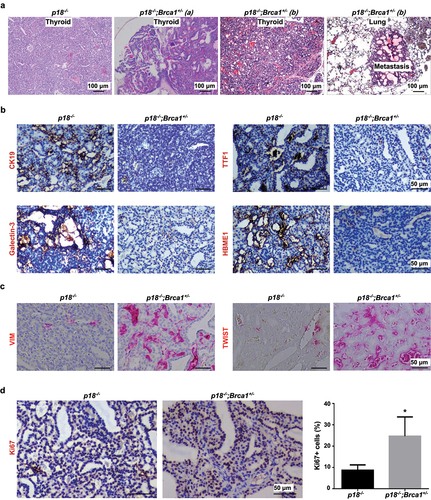
Table 1 Spontaneous thyroid tumor development in mutant mice a
Table 2. Haploid loss of Brca1 in p18-deficient mice activates EMT in thyroid tumor cells.
Figure 3. Overexpression of BRCA1 in human thyroid cancer cells inhibits EMT. 8505C cells transfected with pBabe-empty (empty) and pBabe-HA-BRCA1 (BRCA1) were analyzed by Western blot (a) or qRT-PCR (b). GAPDH was used as a loading control in Western blot.
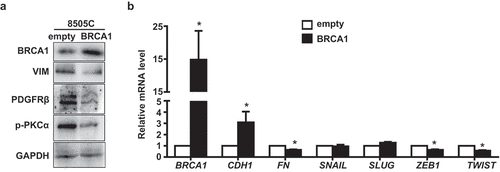
Figure 4. Knockdown of BRCA1 in human thyroid cancer cells promotes EMT and dedifferentiation. B-CPAP (a, b, c) and 8505C (d, e, f) cells infected with pGIPZ-sh-Control (sh-Ctrl) or pGIPZ-sh-BRCA1 targeting different sequences of human BRCA1 (sh-BRCA1-G6 and sh-BRCA1-B7) were analyzed by Western blot (a, d) or qRT-PCR (c, f). * p < 0.05 between the sh-Ctrl group and sh-BRCA1-G6 group, or between the sh-Ctrl group and sh-BRCA1-B7 group. (b, e) The protein levels of each lane in (a) and (d) were quantified, respectively, by Image-Pro Plus 6.0 and normalized by that of GAPDH.
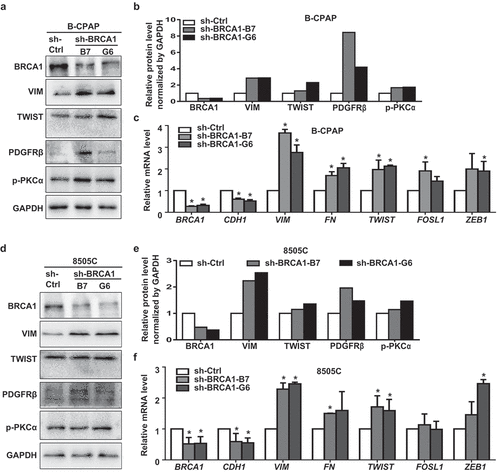
Figure 5. Knockdown of BRCA1 in human thyroid cancer cells promotes EMT and tumorigenesis. (a, b) B-CPAP-sh-Ctrl and B-CPAP-sh-BRCA1-G6 cells were inoculated into the left and right back of four NSG mice, respectively, in a pairwise manner. Similarly, B-CPAP-sh-Ctrl and B-CPAP-sh-BRCA1-B7 cells were inoculated into the left and right back of two NCG mice, respectively, in a pairwise manner. In addition, the same number of B-CPAP-sh-BRCA1-B7 cells were inoculated into the left back of two individual mice. Gross appearance (a) and weight (b) of the tumors formed 5 weeks later were analyzed. Data in (b) represent the mean ± SD of four tumors in each sh-BRCA1 group and six tumors in sh-Ctrl group. * p < 0.05 between the sh-Ctrl group and sh-BRCA1-G6 group, or between the sh-Ctrl group and sh-BRCA1-B7 group. (c, d) Representative tumors derived from sh-Ctrl and sh-BRCA1-B7 cell transplants were analyzed by IHC (c) and Western blot (d). (e) The protein levels of each lane in (d) were quantified by Image-Pro Plus 6.0 and normalized by that of GAPDH (right). Results represent the mean ± SD of four tumors per group.
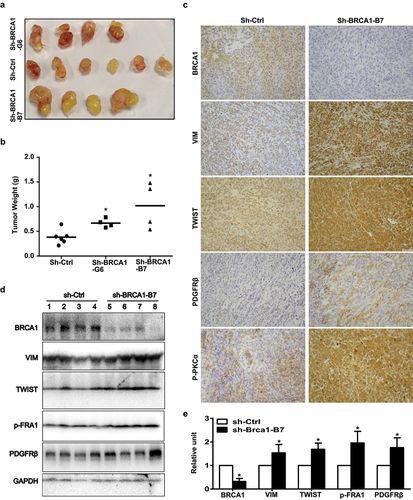
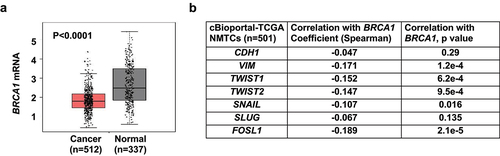
Figure 6. Analysis of BRCA1 expression and its correlation with EMT markers in human thyroid tissues and cancers. (a) Analysis of BRCA1 mRNA expression in thyroid cancer samples (Cancer) and paired normal thyroid tissues (Normal) in GEPIA (http://gepia.cancer-pku.cn) dataset. (b) Correlation analysis of mRNA expression of BRCA1 and EMT markers in thyroid carcinoma/NMTC samples (TCGA, Firehose Legacy) by cBioportal for Cancer Genomics (https://www.cbioportal.org).
Supplemental Material
Download PDF (639.7 KB)Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. All data generated/analyzed during the current study are available from the corresponding auther upon request.
