Figures & data
Table 1 Demographics and baseline disease.
Figure 2 Mean change in FEV1 from baseline over the 24-hour dosing interval after the first dose at Week 0 (A) and at Week 12 (B). There was substantial bronchodilation relative to placebo for the active treatments over the 24-hour interval and at the 24-hour trough at both time points.
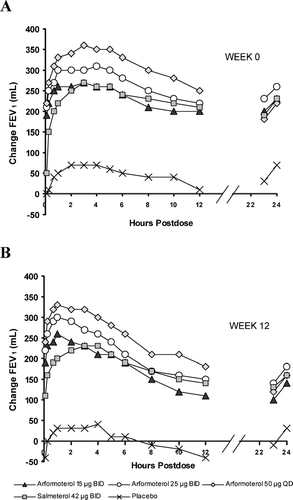
Table 2 Percent change in trough FEV1.
Table 3 Percent change in FEV1 area under the curve over 12 hours (FEV1 AUC(0 - 12 hrs).
Table 4 Peak percent change in FEV1.
Table 5 Percentage of responders and median time to onset of response
Figure 3 Subgroup analysis of the percent change in morning trough FEV1 (left column) and the percent change in FEV1 AUC(0 - 12 hrs) (right column) by baseline percent predicted FEV1, percent reversibility, steroid-use. Subjects with more severe FEV1 compromise or greater FEV1 reversibility at baseline had greater percent improvement in lung function than those with less severe or less reversible disease. The active treatments improved trough FEV1 and FEV1 AUC(0 - 12 hrs) to a greater extent than placebo regardless of baseline corticosteroid use.
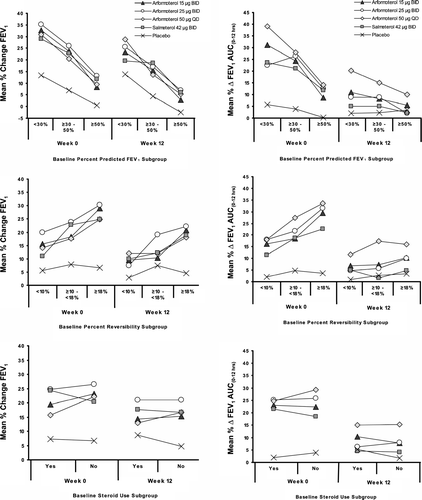
Table 6 Safety summary.