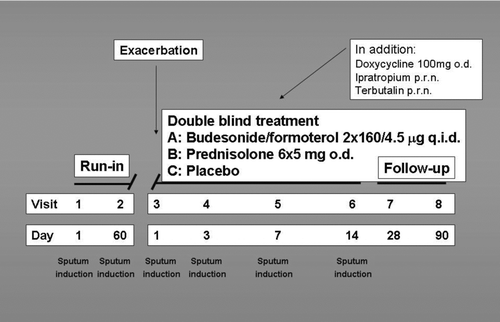
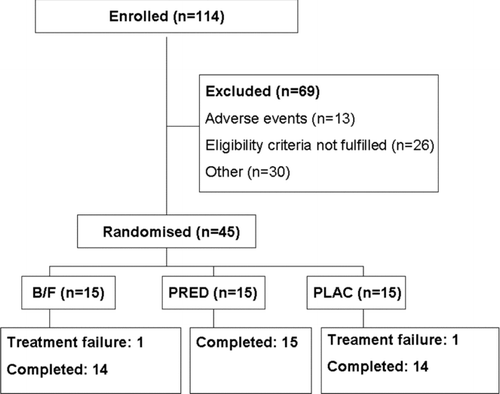
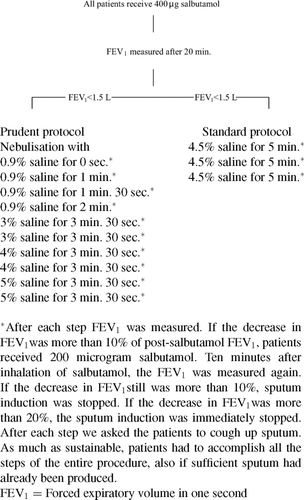
Figures & data
Table 1 Patient characteristics
Figure 3 * On day 3, 7, and 14, the ratio of sputum eosinophil % of the visit under study to the eosinophil% at randomisation are presented. The difference in ratios from start to end of treatment are significant for budesonide/formoterol versus placebo (p = 0.01) and for prednisolone versus placebo (p = 0.007). Data is expressed as geometric means and standard error of the mean. P-values for comparisons of these ratios at day 14 under budesonide/formoterol (320/9 μ g 4 times daily) versus prednisolone (30 mg once daily) and placebo.
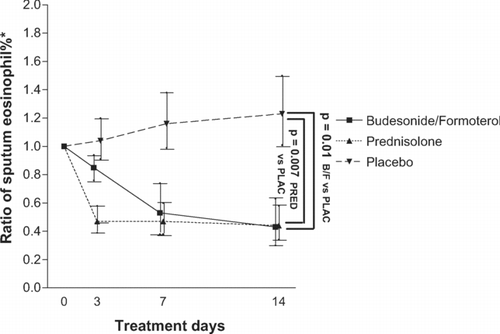
Table 2 Sputum cells
Table 3 mRNA expression of sputum cells
Figure 4 *On day 3, 7, and 14, the ratio of FEV1 of the visit under study to the FEV1 at randomisation are presented. Data is expressed as geometric means and standard error of the mean. P-values for comparisons of these ratios at day 14 under budesonide/formoterol (320/9 μ g 4 times daily) versus prednisolone (30 mg once daily) and placebo.
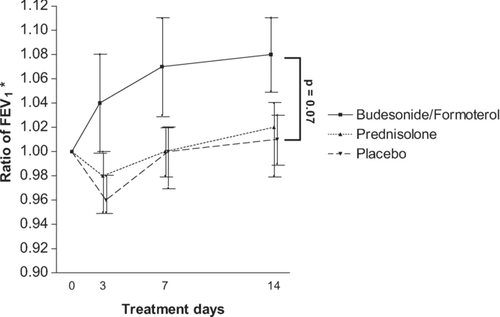
Table 4 Lung function parameters
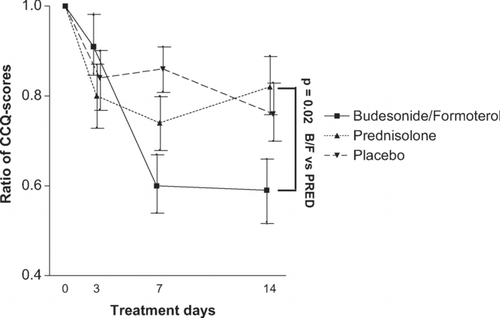
Figure 5 *On day 3, 7, and 14, the difference of CCQ-score of the visit under study from the CCQ-score at randomisation are presented. The difference in means from start to end of treatment are significant for budesonide/formoterol versus prednisolone (p = 0.02) Data is expressed as means and standard error of the mean. P-values for comparisons of the arithmetic mean changes at day 14 under budesonide/formoterol (320/9 μ g 4 times daily) versus prednisolone (30 mg once daily) and placebo.