Figures & data
Table 1. The contents of human neutrophil granules and secretory vesicles.
Figure 1. Phagosome maturation.
The neutrophil NADPH oxidase machinery, activated by delivery of its membrane-bound components to the phagosome, pumps electrons into the phagosomal space to generate toxic ROS. Membrane trafficking allows delivery of primary and secondary granules to the phagosomal membrane, which release a variety of microbicidal proteins into the phagosomal space. MPO released from primary granules reacts with ROS to further produce highly toxic substances. Adapted from (Citation161).
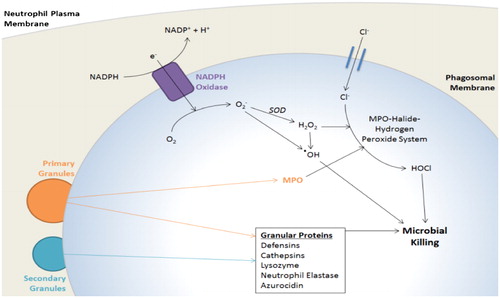
Table 2. The actions of neutrophil elastase.
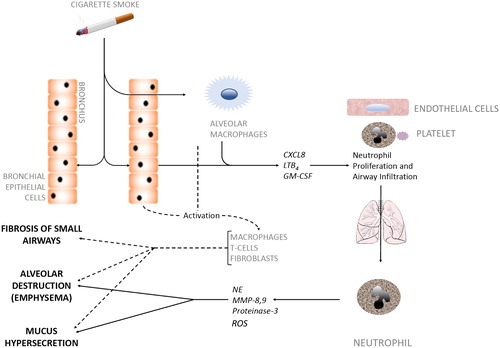
Figure 2. Cellular interactions in COPD pathogenesis.
Inhaled irritants such as cigarette smoke stimulate bronchial epithelial cells and alveolar macrophages to secrete factors (CXCL8, LTB4, GM-CSF) that promote both neutrophil production in bone marrow and neutrophil migration towards inflamed airways, a process coordinated by activated platelets, endothelial cells and the neutrophil. Neutrophils infiltrate the airways in large numbers and produce serine proteases and elastolytic enzymes (e.g. NE, MMP-8,9, proteinase-3). These neutrophilic enzymes act alongside other activated immune cells (dotted line) to degrade alveolar tissue leading to emphysema, over-stimulate Goblet cells leading to hypersecretion of mucus, and cause fibrosis of the small airways, all of which are hallmark features of COPD. CXCL8, Chemokine (CXC motif) ligand 8; LTB4, Leukotriene B4; GM-CSF, Granulocyte-Macrophage Colony-Stimulating Factor; NE, Neutrophil Elastase; MMP, Matrix Metalloproteases.