Figures & data
Figure 1. Metabolic and ventilatory responses to exercise. (a) oxygen uptake (V′O2), (b) minute ventilation (V′E)/carbon dioxide production (V′CO2), (c) V′E, (d) respiratory frequency (fR), (e) tidal volume (VT) and (f) operating lung volumes at different work rates in patients with mild COPD and healthy controls. Triangles represent the VT/V′E inflection. Data are presented as mean ± standard error of the mean, *p<.05. EELV: end-expiratory lung volume; EILV: end-inspiratory lung volume; fR: respiratory frequency; TLC: total lung capacity; V′CO2: carbon dioxide production; V′E: minute ventilation; V′O2: oxygen uptake; VT: tidal volume. Reproduced with permission of the © ERS 2019: European Respiratory Journal 44 (5) 1177–1187; DOI:10.1183/09031936.00034714 Published 31 October 2014.
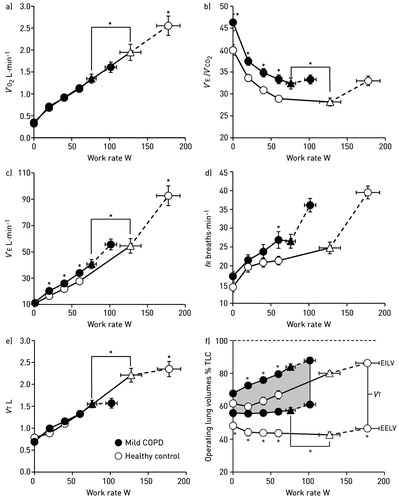
Figure 2. Tidal volume (VT)/dynamic inspiratory capacity (IC) ratio as a function of ventilation during constant work rate exercise in COPD patients showing progressively lower FEV1 values [quartile (Q) 1 to 4] (A). Dyspnea is expressed relative to VT/IC and ventilation (absolute and relative to peak values) in (B), (C) and (D), respectively. Note the clear inflection (plateau) in the VT/ventilation relationship (A), which coincides with a simultaneous inflection in dyspnea (B) across the FEV1 quartiles. FEV1: forced expiratory volume in 1 s; IC: inspiratory capacity; VT: tidal volume. Reprinted from Chest, 141(3), O'Donnell DE, Guenette JA, Maltais F, Webb KA, Decline of resting inspiratory capacity in COPD the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise, page 760, Copyright (2012), with permission from Elsevier.
![Figure 2. Tidal volume (VT)/dynamic inspiratory capacity (IC) ratio as a function of ventilation during constant work rate exercise in COPD patients showing progressively lower FEV1 values [quartile (Q) 1 to 4] (A). Dyspnea is expressed relative to VT/IC and ventilation (absolute and relative to peak values) in (B), (C) and (D), respectively. Note the clear inflection (plateau) in the VT/ventilation relationship (A), which coincides with a simultaneous inflection in dyspnea (B) across the FEV1 quartiles. FEV1: forced expiratory volume in 1 s; IC: inspiratory capacity; VT: tidal volume. Reprinted from Chest, 141(3), O'Donnell DE, Guenette JA, Maltais F, Webb KA, Decline of resting inspiratory capacity in COPD the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise, page 760, Copyright (2012), with permission from Elsevier.](/cms/asset/76be2350-c2da-4d9d-9974-360cf4caa10a/icop_a_1606189_f0002_b.jpg)
Figure 3. Shallower slopes of exertional breathlessness (A) and leg discomfort (B) over time during constant-load cycle exercise after exercise training (EXT) or control intervention in patients with COPD. Endurance time also increased significantly after EXT. Slopes of carbon dioxide output (VCO2) (C) ventilation (VE) (D) and breathing frequency (F) also fell significantly after EXT with no significant changes in tidal volume (VT) (E), *p<.05. EXT: exercise training; F: respiratory frequency; V′CO2: carbon dioxide production; V′E: minute ventilation; VT: tidal volume. Reprinted with permission of the American Thoracic Society. Copyright © 2019 American Thoracic Society. O'Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med. 1998;157(5):1489–1497. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
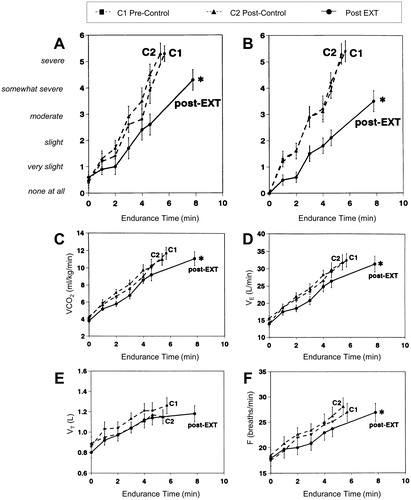
Figure 4. Changes in inspiratory capacity (IC) as a function of time (A) and breathing frequency (B) during constant-load exercise prior to and following an exercise training program in a representative patient with COPD. The dashed arrows connect isotime values. COPD: chronic obstructive pulmonary disease; f: respiratory frequency; IC: inspiratory capacity. Reprinted from Chest, 128(4), Porszasz J, Emtner M, Goto S, Somfay A, Whipp BJ, Casaburi R, Exercise training decreases ventilatory requirements and exercise induced hyperinflation at submaximal intensities in patients with COPD, page 2030, Copyright (2005), with permission from Elsevier.
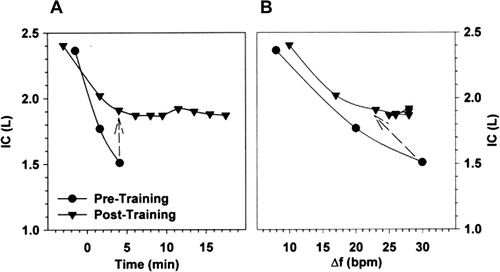
Figure 5. Change in resting maximal inspiratory and expiratory pressure (MIP and MEP, respectively) and inspiratory muscle endurance time in COPD patients who completed a general exercise training program compared to control, *p<.05 compared to pre-intervention baseline. COPD: chronic obstructive pulmonary disease; MIP: maximum inspiratory pressure; MEP: maximum expiratory pressure. Reprinted with permission of the American Thoracic Society. Copyright © 2019 American Thoracic Society. O'Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med. 1998;157(5):1489–1497. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
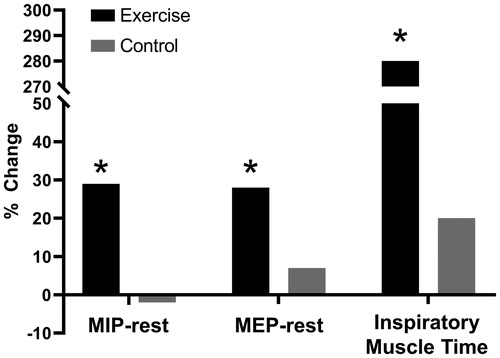
Figure 6. Lower dyspnea intensity (A and D), diaphragm electromyography measured during tidal inspiration/largest value during a maximum inspiratory maneuver (EMGdi/EMGdimax) (B and E), and ventilation (C and F) during constant work rate exercise before and after inspiratory muscle training (IMT) (A–C) and the control intervention (D–F) in patients with COPD showing inspiratory muscle weakness at baseline. Values are mean ± standard error. *p<.05, post- versus pre-intervention at isotime. COPD: chronic obstructive pulmonary disease; IMT: inspiratory muscle training; EMGdi/EMGdimax: diaphragm electromyography measured during tidal inspiration/largest value during a maximum inspiratory maneuver. Adapted from: Langer D, Ciavaglia C, Faisal A, Webb KA, Neder JA, Gosselink R, Dacha S, Topalovic M, Ivanova A, O’Donnell DE. Inspiratory muscle training reduces diaphragm activation and dyspnea during exercise in COPD. J Appl Physiol. 2018;125(2):381–92.
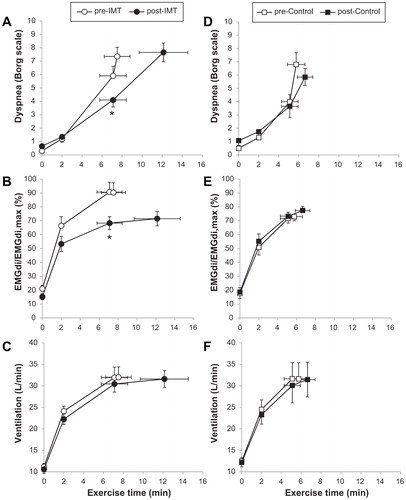
Figure 7. Impact of exercise training as part of pulmonary rehabilitation or control intervention on dyspnea (A), anxiety (B), depression (C) and self-efficacy (D) in patients with COPD. Lack of change in O2 uptake (V′O2) (E and F) and ventilation (V′E) (G and H) during constant-load exercise pre- and post-exercise training as part of pulmonary rehabilitation (EXT) or control intervention (C) in patients with COPD. Error bars represent standard error of the mean, *p<.05. COPD: chronic obstructive pulmonary disease; EXT: pulmonary rehabilitation; HADS: Hospital Anxiety and Depression Scale; TDI: Translational Dyspnea Index; V′O2: oxygen consumption. Adapted from: Wadell K, Webb KA, Preston ME, Amornputtisathaporn N, Samis L, Patelli J, Guenette JA, O’Donnell DE. Impact of pulmonary rehabilitation on the major dimensions of dyspnea in COPD. COPD. 2013;10(4):425–35.
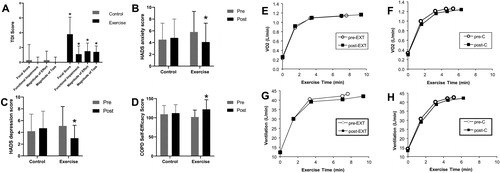
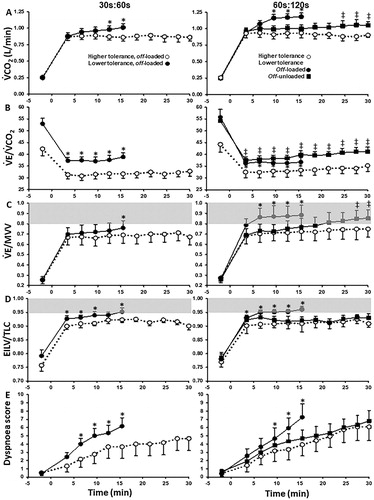
Figure 8. Metabolic (A), ventilatory, (B and C), mechanical (D) and sensory (E) responses to 30s:60s (left) and 60s:120s (right) interval protocols in COPD patients who tolerated or not off-loaded protocols for 30 min (“higher-” and “lower-tolerance”, respectively). The latter group repeated the most demanding protocol (60s:120s) with no load in the recovery phases (off-unloaded). Shaded areas indicate physiological limits typically associated with diminished exercise tolerance in COPD. Values are mean and standard error. *p<.05, between-groups, intra-protocol comparisons; †60s:120 s off-unloaded versus off-loaded in the “lower-tolerance” group. EILV: end inspiratory lung volume; MVV: maximal voluntary ventilation; V′CO2: carbon dioxide output; V′E: minute ventilation; TLC: total lung capacity. Reprinted from Respiratory Physiology & Neurobiology, 250, Bravo DM, Gimenes AC, Amorim BC, Alencar MC, Berton DC, O'Donnell DE, Nery LE, Neder JA, Excess ventilation in COPD implications for dyspnoea and tolerance to interval exercise, page 11, Copyright (2018), with permission from Elsevier.