Figures & data
Figure 1. Flow chart depicting sample selection. a: Full coverage and COPD diagnosis <1 year prior to first nebulized LABA claim. Excluded deaths before first nebulized LABA claims. COPD: chronic obstructive pulmonary disease; LABA: long-acting beta2 agonist; SNF: skilled nursing facility; ESRD: end stage renal disease.
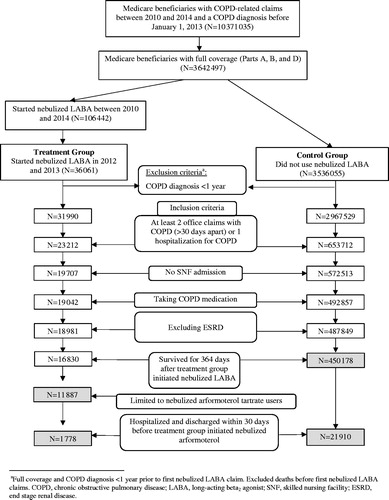
Table 1. Sociodemographic characteristics of nebulized arformoterol users and controls.
Figure 2. Top 10 most common COPD treatments among nebulized arformoterol users and controls in the 12 months prior to initiation of nebulized LABA (for the study group) or the assigned start date (for controls). COPD: chronic obstructive pulmonary disease; CS: corticosteroids; SABD: short-acting bronchodilator; ICS: inhaled corticosteroids; LABA: long-acting beta2 agonist; SABA: short-acting beta2 agonists; LAMA: long-acting muscarinic antagonist; AC: anticholinergics; *p < .05.
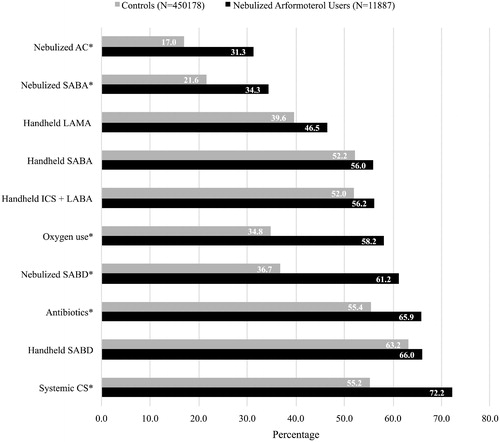
Figure 3. a: Exacerbations and health resource utilization patterns in the 12 months prior to initiation of nebulized LABA (for the study group) or the assigned start date (for controls). ICU: intensive care unit; COPD: chronic obstructive pulmonary disease; ED: emergency department; * p < .05. b: Exacerbations and health resource utilization patterns in the 12 months prior to initiation of nebulized LABA (for the study group) or the assigned start date (for controls) among Medicare beneficiaries with recent hospitalizations. ED: emergency department; ICU: intensive care unit; COPD: chronic obstructive pulmonary disease; * p < .05.
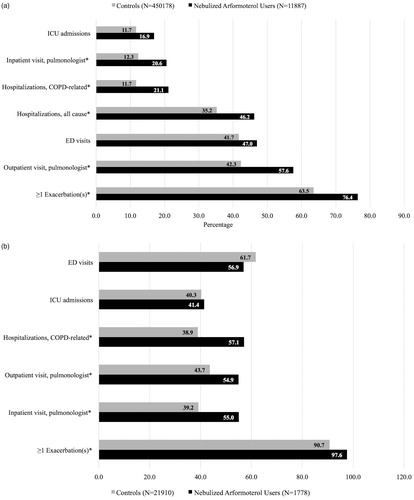
Figure 4. a. Average number of exacerbations, health resources used, and comorbidity in the 12 months prior to initiation of nebulized LABA (for the study group) or the assigned start date (for controls). COPD: chronic obstructive pulmonary disease; ED: emergency department. b. Average number of exacerbations, health resources used, and co-morbidities in the 12 months prior to initiation of nebulized LABA (for the study group) or the assigned start date (for controls) among Medicare beneficiaries with recent hospitalizations. COPD: chronic obstructive pulmonary disease; LOS: length of stay; ED: emergency department.
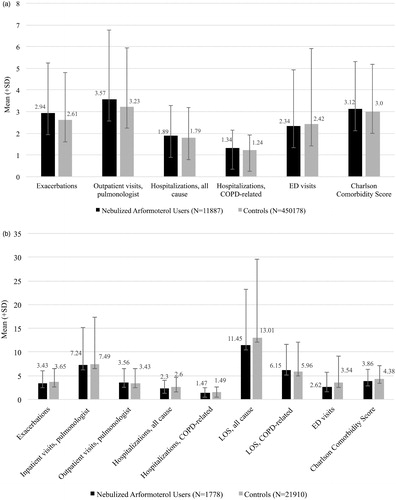
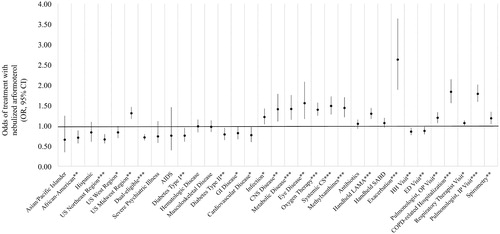
Figure 5. Predictors of nebulized arformoterol treatment among Medicare beneficiaries with COPD. COPD: chronic obstructive pulmonary disease; OR: odds ratio; CI: confidence interval; US: United States; AIDS: acquired immune deficiency syndrome; GI: gastrointestinal; CNS: central nervous system; CS: corticosteroids; LAMA: long-acting muscarinic antagonist; SABD: short-acting bronchodilator; HH: home healthcare; ED: emergency department; OP: outpatient; IP: inpatient; * p < .05; ** p < .01; *** p < .001.
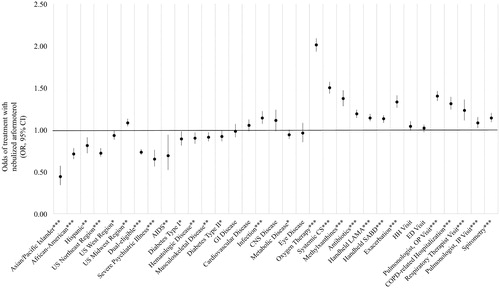
Figure 6. Predictors of nebulized arformoterol treatment among Medicare beneficiaries with recent hospitalizations. COPD: chronic obstructive pulmonary disease; OR: odds ratio; CI: confidence interval; US: United States; AIDS: acquired immune deficiency syndrome; GI: gastrointestinal; CNS: central nervous system; CS: corticosteroids; LAMA: long-acting muscarinic antagonist; SABD: short-acting bronchodilator; HH: home healthcare; ED: emergency department; OP: outpatient; IP: inpatient; *p<.05; **p<.01; ***p<.001.