Figures & data
Table 1. This is currently in the middle of the introduction - please could it be moved to the next page, closer to where it's first cited.
Figure 1. (a) Change in trough FEV1 at Week 12 from baseline; all patients and by GOLD status. (b) Change in trough FEV1 at Week 12 from baseline; by baseline SGRQ and BDI status.
BDI, Baseline Dyspnea Index; CI, confidence interval; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SE, standard error; SGRQ, St. George’s Respiratory Questionnaire; T/O, tiotropium/olodaterol; Tio, tiotropium.
N numbers below bars, adjusted mean response within bars.
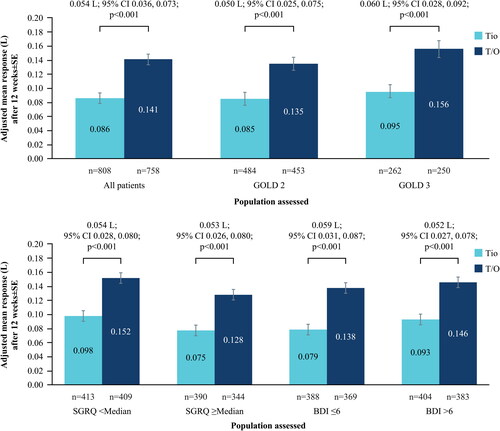
Figure 2. (a) Change in SGRQ at Week 12 from baseline; all patients and by GOLD status. (b) Change in SGRQ at Week 12 from baseline; by baseline SGRQ and BDI status.
BDI, Baseline Dyspnea Index; CI, confidence interval; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SE, standard error; SGRQ, St. George’s Respiratory Questionnaire; T/O, tiotropium/olodaterol; Tio, tiotropium.
N numbers below bars, adjusted mean response within bars.
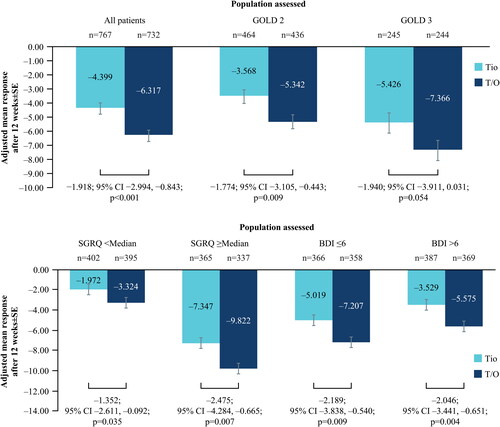
Figure 3. (a) Change in TDI at Week 12 from baseline; all patients and by GOLD status. (b) Change in TDI at Week 12 from baseline; by baseline SGRQ and BDI status.
BDI, Baseline Dyspnea Index; CI, confidence interval; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SE, standard error; SGRQ, St. George’s Respiratory Questionnaire; T/O, tiotropium/olodaterol; TDI, Transition Dyspnea Index; Tio, tiotropium.
N numbers below bars, adjusted mean response within bars.
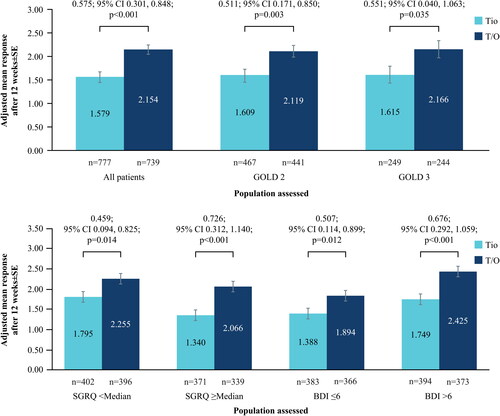
Table 2. Adverse event profile.
Supplemental Material
Download PDF (669.4 KB)Data availability
The data that support the findings of this study are available from the corresponding author, PMAC, upon reasonable request.
