Figures & data
Figure 1. Schematic of two-step study design. The idealized single-path model is shown in an extended view without considering the gravity angle.
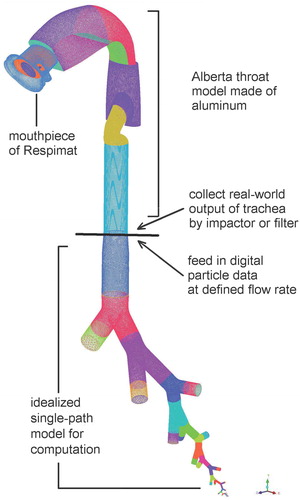
Figure 2. Experimental set-up for measuring deposition in the mouth–throat model (Alberta throat). NGI: Next Generation Impactor; RH: relative humidity.
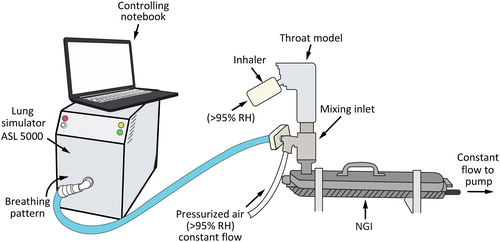
Figure 3. Dose to lung collected at the outlet of the Alberta throat model. Error bars are standard deviation. COPD: chronic obstructive pulmonary disease; ND: nominal dose; OLO: olodaterol in the fixed-dose combination; TIO: tiotropium in the fixed-dose combination; TIO/OLO: tiotropium/olodaterol fixed-dose combination.
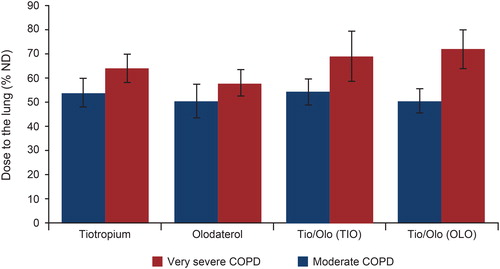
Figure 4. Cumulative particle size distribution, measured by NGI at the outlet of the Alberta throat model (very severe COPD). COPD: chronic obstructive pulmonary disease; FPF: fine particle fraction; MMAD: mass median aerodynamic diameter; NGI: Next Generation Impactor; OLO: olodaterol in the fixed-dose combination; TIO: tiotropium in the fixed-dose combination; TIO/OLO: tiotropium/olodaterol fixed-dose combination.
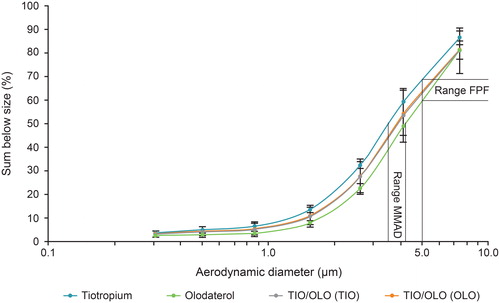
Figure 5. Fine particle fraction (<5 µm) in percent of the nominal dose measured at the outlet of the Alberta throat model. Error bars are standard deviations. COPD: chronic obstructive pulmonary disease; FPF: fine particle fraction; ND: nominal dose; OLO: olodaterol in the fixed-dose combination; TIO: tiotropium in the fixed-dose combination; TIO/OLO: tiotropium/olodaterol fixed-dose combination.
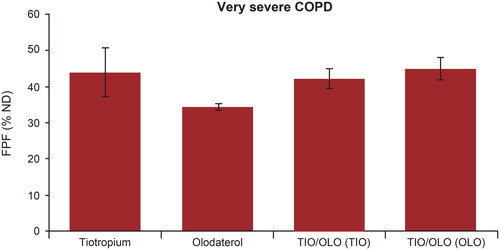
Figure 6. Regional deposition of particles in an adult lung model using a three-dimensional single-path simulation under flow rates of a) 27.5 L/min, b) 55 L/min and c) 82.5 L/min (reference: dose to lung, very severe COPD). G0–G23 represent generations of the lung branchings. With the exception of the first branching, the model assumes an equal flow separation into two daughter branches. The error bars have been calculated based on error propagation of experimental data (input variability from the particle size distributions of ). COPD: chronic obstructive pulmonary disease; DTL: dose to lung; OLO: olodaterol in the fixed-dose combination; TIO: tiotropium in the fixed-dose combination; TIO/OLO: tiotropium/olodaterol fixed-dose combination.
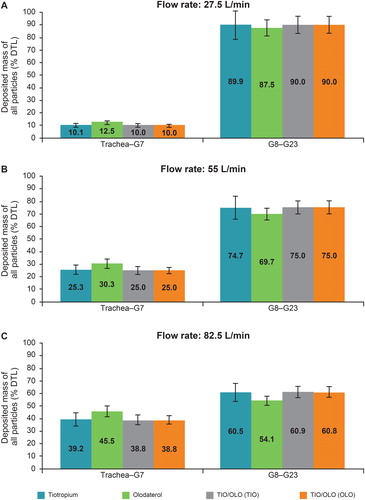
Figure 7. Typical scintigraphic images for Respimat® SMI™, Turbohaler® DPI at slow inhaled flow rate, Turbohaler® DPI with fast inhaled flow rate, and Becloforte® pMDI. Reprinted with permission from Pitcairn et al. [Citation20]. Copyright © 2005. The publisher for this copyrighted material is MaryAnn Liebert, Inc. publishers. DPI: dry powder inhaler; pMDI: pressurized metered-dose inhaler; SMI: Soft MistTM Inhaler.
![Figure 7. Typical scintigraphic images for Respimat® SMI™, Turbohaler® DPI at slow inhaled flow rate, Turbohaler® DPI with fast inhaled flow rate, and Becloforte® pMDI. Reprinted with permission from Pitcairn et al. [Citation20]. Copyright © 2005. The publisher for this copyrighted material is MaryAnn Liebert, Inc. publishers. DPI: dry powder inhaler; pMDI: pressurized metered-dose inhaler; SMI: Soft MistTM Inhaler.](/cms/asset/d54ef4d6-3cf7-4cf0-9e87-07e1590fce8e/icop_a_1853091_f0007_c.jpg)
Supplemental Material
Download PDF (709.4 KB)Supplemental Material
Download PDF (92.4 KB)Data availability statement
The data used and analyzed during the current study are available from the corresponding author on reasonable request.
