Figures & data
Figure 1. Subject disposition.
*The protocol permitted subjects to be screened multiple times. †One subject randomized to GB 25 µg BID withdrew prior to receiving study medication, and so was excluded from the safety and ITT populations. GB, glycopyrronium bromide; BID, twice daily; QD, once daily; ITT, intention-to-treat.
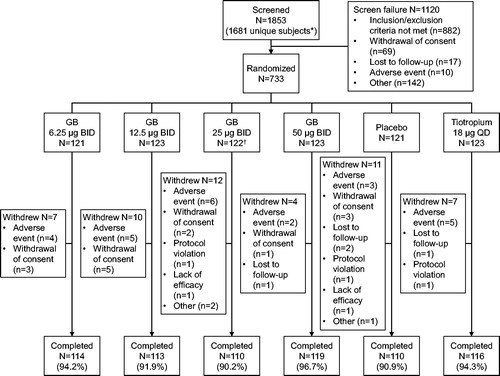
Table 1. Subject demographics and disease characteristics at screening (ITT population).
Figure 2. FEV1 AUC0–12h on Day 1 and at Week 6 (ITT population).
Bars are adjusted mean changes from baseline, with 95% confidence intervals (*95% CI and p values adjusted for multiplicity). Values are adjusted mean treatment–placebo differences, 95% confidence intervals and p values. †p < 0.05 vs GB 6.25 µg BID; ‡p < 0.05 vs GB 12.5 µg BID. Tiotropium–GB differences were not analyzed. ITT, intention-to-treat; GB, glycopyrronium bromide; BID, twice daily; QD, once daily; FEV1, forced expiratory volume in 1 second; AUC, area under the curve.
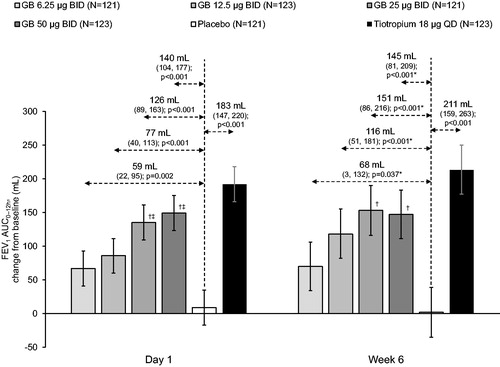
Figure 3. Pre-dose morning FEV1 at Weeks 3 and 6 (ITT population).
Bars are adjusted mean changes from baseline, with 95% confidence intervals. Values are adjusted mean treatment–placebo differences, 95% confidence intervals and p values. †p < 0.05 vs GB 6.25 µg BID. Tiotropium–GB differences were not analyzed. ITT, intention-to-treat; GB, glycopyrronium bromide; BID, twice daily; QD, once daily; FEV1, forced expiratory volume in 1 second.
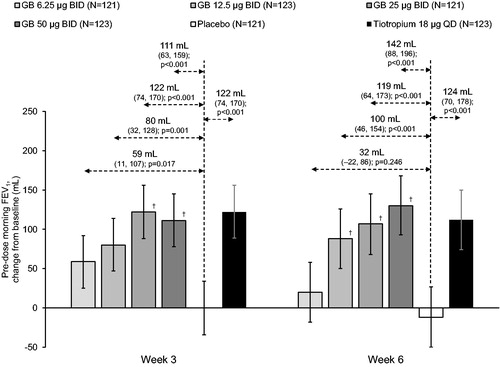
Figure 4. TDI focal score at Weeks 3 and 6 (ITT population).
Bars are adjusted mean, with 95% confidence intervals. Values are adjusted mean treatment–placebo differences, 95% confidence intervals and p values. †p < 0.05 vs GB 6.25 µg BID. Tiotropium–GB differences were not analyzed. ITT, intention-to-treat; GB, glycopyrronium bromide; BID, twice daily; QD, once daily; TDI, Transition Dyspnea Index.
Figure 5. E-RS total score, analyzed across the study duration (ITT population).
There were no significant differences between GB doses. Tiotropium–GB differences were not analyzed. ITT, intention-to-treat; GB, glycopyrronium bromide; BID, twice daily; QD, once daily; E-RS, Evaluating Respiratory Symptoms questionnaire.
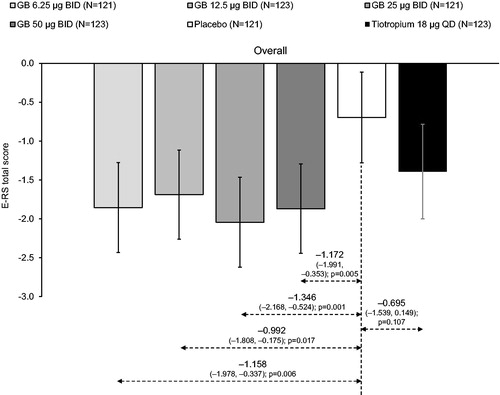
Table 2. TDI focal score responder analysis at Weeks 3 and 6 (ITT population).
Table 3. Adverse events, overall and most common preferred terms (≥2% of subjects in any group; safety population).
Table 4. QTc (Fridericia’s correction) interval data at any post-dose timepoint (Safety population).
Supplemental Material
Download PDF (430.1 KB)Data availability
Chiesi commits to sharing with qualified scientific and medical Researchers, conducting legitimate research, patient-level data, study-level data, the clinical protocol and the full clinical study report of Chiesi Farmaceutici S.p.A.-sponsored interventional clinical trials in patients for medicines and indications approved by the European Medicines Agency and/or the US Food and Drug Administration after 1st January 2015, following the approval of any received research proposal and the signature of a Data Sharing Agreement. Chiesi provides access to clinical trial information consistently with the principle of safeguarding commercially confidential information and patient privacy. To date, the current study is out of scope of the Chiesi policy on Clinical Data Sharing. Other information on Chiesi’s data sharing commitment, access and research request’s approval process are available in the Clinical Trial Transparency section of http://www.chiesi.com/en/research-and-development/.
