Figures & data
Table 1. Baseline characteristics of the study cohort of 29,716 initiators of fluticasone-based and 9,646 initiators of budesonide-based triple therapy, after fine stratification weighting from probability of treatment propensity scores, with corresponding standardized mean differences.
Figure 1. One-year cumulative incidence of moderate-severe exacerbation (a) and severe exacerbation (b), comparing fluticasone-based with budesonide-based triple therapy, estimated using the Kaplan-Meier method, after adjustment by fine stratification weights from the probability of treatment propensity scores.
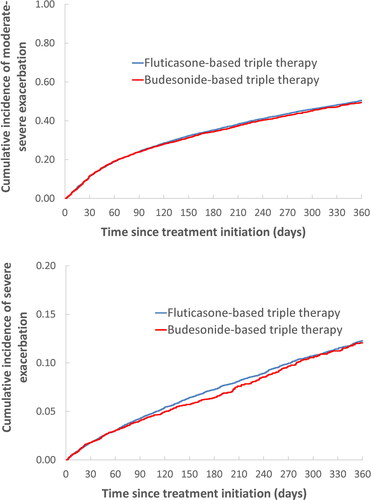
Figure 2. One-year cumulative incidence of all-cause death (a) and severe pneumonia (b), comparing fluticasone-based with budesonide-based triple therapy, estimated using the Kaplan-Meier method, after adjustment by fine stratification weights from the probability of treatment propensity scores.
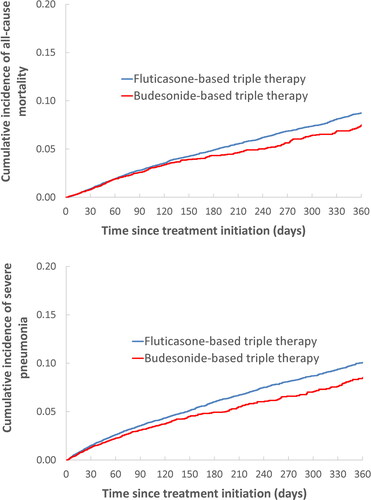
Table 2. Adjusted hazard ratios of COPD exacerbation, all-cause mortality and severe pneumonia requiring hospitalization comparing fluticasone-based with budesonide-based triple therapy in COPD patients in the first year after initiation, from the as-treated analysis.
Table 3. Adjusted hazard ratios of moderate or severe exacerbation comparing fluticasone-based with budesonide-based triple therapy initiation in patients with COPD, from the as-treated analyses over one-year follow-up, stratified by prior asthma, prior exacerbations and peripheral blood eosinophil count.
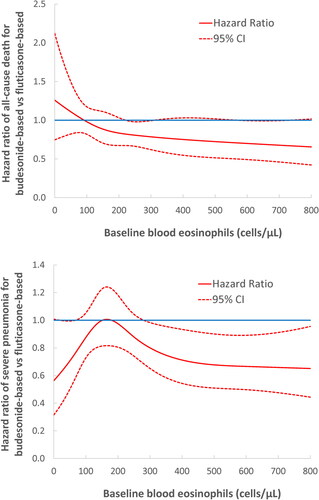
Figure 3. Adjusted hazard ratio (solid line) of all-cause death (a) and severe pneumonia (b) comparing fluticasone-based with budesonide-based triple therapy and 95% confidence intervals (dashed lines) according to blood eosinophil count (cells/µL) prior to treatment initiation, from the as-treated analysis fit by cubic splines.
Supplemental Material
Download ()Data availability statement
This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the UK National Health Service as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. Because electronic health records are classified as “sensitive data” by the UK Data Protection Act, information governance restrictions (to protect patient confidentiality) prevent data sharing via public deposition. Data are available with approval through the individual constituent entities controlling access to the data. Specifically, the primary care data can be requested via application to the Clinical Practice Research Datalink (https://www.cprd.com).
