Figures & data
Figure 1. Study design. People aged 66 and above who newly started triple theray from 2014 to 2017 were identified and their prior treatment regemins were traced unitil their COPD dianosis. However, the earlist year for tracing year was 2003 as all the three composite of triple therapy became avaiable in market in 2013.
Abbreviations: COPD, chronic obstructive pulmonary disease, ICS, inhaled corticosteroid; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist.
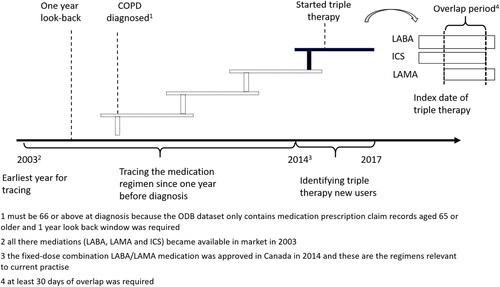
Figure 2. Flow chart of study population selection. People were deemed to be on triple therapy if supply of the three components (LABA, LAMA, and ICS, either in free-dose or in fixed-dose combination) overlapped for at least 30 consecutive days. We excluded patients with a co-diagnosis of asthma on or before the index date and/or who were ineligible for drug benefits. We also removed those diagnosed before January 1, 2003, as LAMAs were introduced to the Canadian market in 2003 and if LAMA were missing among those peoples’ medications it could have been because it was unavailable. Those who received a prescription for a LABA, LAMA and/or ICS use prior to diagnosis were also excluded because of concern the diagnosis date was not accurate.
Abbreviations: COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist.
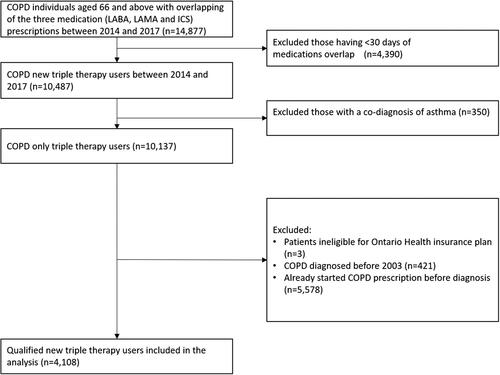
Table 1. Selected baseline characteristics of COPD patients who initiated triple therapy in Ontario, Canada between 2014 and 2017 who had full treatment pathway available since diagnosis in or after 2003.
Figure 3. Distribution of medication pathways progress preceding initiation of triple therapy (ICS + LAMA + LABA) among COPD patients. We only extracted medication records of long-acting maintenance pharmacotherapies (LABA, LAMA and ICS) to examine medication escalation to triple therapy starting after diagnosis. Short-acting bronchodilators were not included as they were considered rescue inhalers that were used as needed, and not as long-acting maintenance.
Abbreviations: COPD, chronic obstructive pulmonary disease, ICS, inhaled corticosteroid; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist.
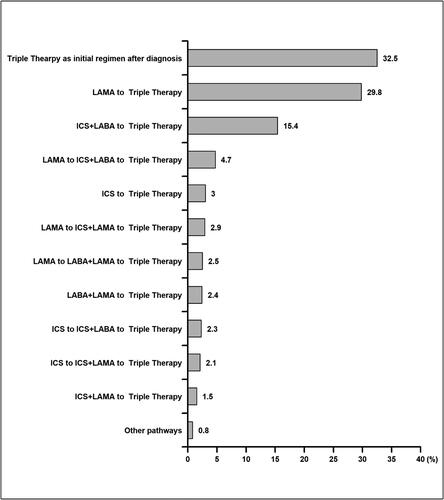
Table 2. Medication progression pathways from diagnosis to triple therapy stratified by exacerbation history, index year and blood eosinophil count.
Data availability statement
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g. healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (email: [email protected]). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
