Figures & data
Figure 1. Selected ventilatory and gas exchange responses to incremental cardiopulmonary exercise testing. Proportional decreases in dead space (VD)/tidal volume (VT) ratio (i.e. the physiological dead space) (a) and ventilation (V̇E)/carbon dioxide output (V̇CO2) (b) ratios maintain arterial carbon dioxide partial pressure (PaCO2) close to resting value during mild-to-moderate exercise (c). The V̇E/V̇CO2 response contour is established by both the slope and the intercept of the linear V̇E-V̇CO2 relationship (d). V̇E increases out of proportion to V̇CO2 after the respiratory compensation point (RCP) (b–d) leading to respiratory alkalosis (c) to compensate for progressive lactic acidemia. Note the increases in the lowest V̇E/V̇CO2 when the lactate threshold is reached at a low exercise intensity, i.e. before the stabilization of V̇E/V̇CO2 at the “true” nadir. Given eucapnia during exercise in most dyspneic patients with mild COPD, their high V̇E-V̇CO2 largely reflects a high physiological dead space, i.e. wasted ventilation. Reproduced, with permission of the publisher, from: Neder et al. [Citation34].
![Figure 1. Selected ventilatory and gas exchange responses to incremental cardiopulmonary exercise testing. Proportional decreases in dead space (VD)/tidal volume (VT) ratio (i.e. the physiological dead space) (a) and ventilation (V̇E)/carbon dioxide output (V̇CO2) (b) ratios maintain arterial carbon dioxide partial pressure (PaCO2) close to resting value during mild-to-moderate exercise (c). The V̇E/V̇CO2 response contour is established by both the slope and the intercept of the linear V̇E-V̇CO2 relationship (d). V̇E increases out of proportion to V̇CO2 after the respiratory compensation point (RCP) (b–d) leading to respiratory alkalosis (c) to compensate for progressive lactic acidemia. Note the increases in the lowest V̇E/V̇CO2 when the lactate threshold is reached at a low exercise intensity, i.e. before the stabilization of V̇E/V̇CO2 at the “true” nadir. Given eucapnia during exercise in most dyspneic patients with mild COPD, their high V̇E-V̇CO2 largely reflects a high physiological dead space, i.e. wasted ventilation. Reproduced, with permission of the publisher, from: Neder et al. [Citation34].](/cms/asset/d59d48f0-67dd-453b-ab0d-792cf95bdacf/icop_a_2301549_f0001_c.jpg)
Figure 2. Putative mechanisms by which the structural abnormalities associated with COPD development may increase alveolar ventilation (V̇a)/capillary perfusion (Q̇c) relationship, increasing the wasted ventilation in the physiological dead space. The relative contribution of these abnormalities vary substantially amongst patients with similar spirometric findings justifying the use of more elaborated pulmonary function tests in addition to imaging to improve disease phenotyping. Reproduced, with permission of the American Thoracic Society. Copyright © 2023 American Thoracic Society. All rights reserved. Neder [Citation43]. Annals of the American Thoracic Society is an official journal of the American Thoracic Society.
![Figure 2. Putative mechanisms by which the structural abnormalities associated with COPD development may increase alveolar ventilation (V̇a)/capillary perfusion (Q̇c) relationship, increasing the wasted ventilation in the physiological dead space. The relative contribution of these abnormalities vary substantially amongst patients with similar spirometric findings justifying the use of more elaborated pulmonary function tests in addition to imaging to improve disease phenotyping. Reproduced, with permission of the American Thoracic Society. Copyright © 2023 American Thoracic Society. All rights reserved. Neder [Citation43]. Annals of the American Thoracic Society is an official journal of the American Thoracic Society.](/cms/asset/3d1e8960-381e-4c31-9572-68de900e5e54/icop_a_2301549_f0002_c.jpg)
Figure 3. A putative explanation by which increased physiological dead space (wasted ventilation) and alveolar hyperventilation may trigger exertional dyspnea. The combination of pulmonary fibrosis and emphysema (CPFE) is depicted for illustrative purposes. In the presence of increased (⇑) airspace (alveolar ventilation (V̇a)) relative to capillary perfusion (Q̇c)) (wasted ventilation), less CO2 from the mixed venous blood is unloaded into the right alveolus. Consequently, the alveolar carbon dioxide tension (PACO2) diminishes (⇓) markedly but PCO2 at the end of the capillary (c’) may rise transitorily, subsequently enriching the PCO2 of the arterial blood sensed by the central and/or peripheral chemoreceptors. This may add to the other sources of afferent stimuli to the pontine–medullary respiratory centers (red circle). The inspiratory neural drive (IND) is heightened; accordingly. Of note, IND is also modulated by cortical (black circle) and limbic (blue circle) influences. Excessive ventilation of the left alveolus with preserved V̇a/Q̇c relationship, triggered by increased chemostimulation or other source(s) of additional ventilatory stimuli, leads to alveolar hyperventilation and hypocapnia. A low arterial Pco2 may downward shift the CO2 set-point, impair the cerebrovascular reactivity (CVR) to CO2, and increase the central and peripheral chemoreceptor gains. Hypoxemia (arterial partial pressure for O2 (PaO2)), if present, sums up with these excitatory inputs to further increase the IND in a vicious circle that fuels breathlessness. In mild COPD, the evidence accrued to date does not support a major contribution of hyperventilation and/or low PaO2 to the patient high IND, suggesting a dominant role for the wasted ventilation.
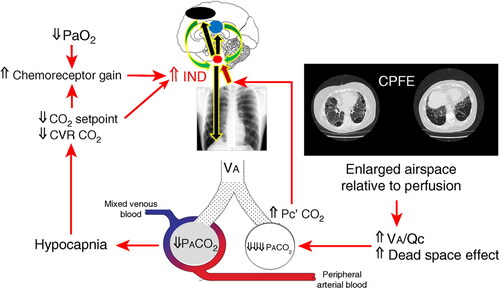
Figure 4. Schematic representation of the key mechanisms by which alveolar ventilation (V̇a)/capillary perfusion (Q̇c) mismatching may conspire to increase the inspiratory neural drive (IND), eventually leading to exertional dyspnea and exercise intolerance in patients with mild COPD. The putative mechanisms that have been ruled out in previous studies are highlighted (reviewed in refs. [Citation9,Citation28]). Emphasis is given to the unknown contribution of impaired perfusion of non-emphysematous lung tissue (in addition to emphysema) to increased (↑) wasted ventilation in the physiological dead space (high dead space (VD)/tidal volume (VT) ratio), heightened dyspnea at given work rate (WR) in these patients was largely commensurate to preserved dyspnea-ventilation (V̇E) relationship, in keeping with the pattern of “excessive breathing” (). See text for further elaboration. Definition of abbreviations: HPV: hypoxic pulmonary vasoconstriction; PAP = pulmonary arterial pressure. Modified, with permission of the publisher, from: Neder et al. [Citation111].
![Figure 4. Schematic representation of the key mechanisms by which alveolar ventilation (V̇a)/capillary perfusion (Q̇c) mismatching may conspire to increase the inspiratory neural drive (IND), eventually leading to exertional dyspnea and exercise intolerance in patients with mild COPD. The putative mechanisms that have been ruled out in previous studies are highlighted (reviewed in refs. [Citation9,Citation28]). Emphasis is given to the unknown contribution of impaired perfusion of non-emphysematous lung tissue (in addition to emphysema) to increased (↑) wasted ventilation in the physiological dead space (high dead space (VD)/tidal volume (VT) ratio), heightened dyspnea at given work rate (WR) in these patients was largely commensurate to preserved dyspnea-ventilation (V̇E) relationship, in keeping with the pattern of “excessive breathing” (Figure 5). See text for further elaboration. Definition of abbreviations: HPV: hypoxic pulmonary vasoconstriction; PAP = pulmonary arterial pressure. Modified, with permission of the publisher, from: Neder et al. [Citation111].](/cms/asset/8cf4823a-83f8-4ed4-9ac7-ee438bcd5f28/icop_a_2301549_f0004_c.jpg)
Figure 5. A simplified framework to expose the major “pulmonary” determinants of exertional dyspnea during incremental cardiopulmonary exercise testing as related to the pathophysiology of COPD. The key physiological abnormalities are highlighted: excess ventilation (high ventilation (V̇E)-(carbon dioxide output (V̇CO2) relationship) and the attainment of critical inspiratory constraints (CIC). In most dyspneic patients with mild COPD, increased wasted ventilation in the physiological dead space (VDphys) prompts a high, V̇E/V̇CO2 ratio leading to a pattern of “excessive breathing” rather than “restrained” (or “constrained”) breathing, i.e. increased dyspnea at given work rate (WR) but within the expected range when expressed relative to the heightened V̇E. Definition of abbreviations and symbols: ⇑: high; ⇓: low’; V̇A: alveolar ventilation; Q̇c: capillary perfusion; Pa: arterial partial pressure; PET: end-tidal partial pressure; Hypervent: alveolar hyperventilation; CIC: end-inspiratory lung volume/total lung capacity≥ 0.9, tidal volume (VT)/inspiratory capacity ≥ 0.7, VT plateau, all reached at an abnormally-low work rate. Ranges of dyspnea severity based on percentiles distribution of scores at a given ventilation as a function of age and sex: 5th–25th: “mild”; 25th–50th: “moderate”; 50th 75th: “severe”; 75th 95th: “very severe” [Citation14]. Reproduced, with permission of the publisher, from: Neder [Citation66].
![Figure 5. A simplified framework to expose the major “pulmonary” determinants of exertional dyspnea during incremental cardiopulmonary exercise testing as related to the pathophysiology of COPD. The key physiological abnormalities are highlighted: excess ventilation (high ventilation (V̇E)-(carbon dioxide output (V̇CO2) relationship) and the attainment of critical inspiratory constraints (CIC). In most dyspneic patients with mild COPD, increased wasted ventilation in the physiological dead space (VDphys) prompts a high, V̇E/V̇CO2 ratio leading to a pattern of “excessive breathing” rather than “restrained” (or “constrained”) breathing, i.e. increased dyspnea at given work rate (WR) but within the expected range when expressed relative to the heightened V̇E. Definition of abbreviations and symbols: ⇑: high; ⇓: low’; V̇A: alveolar ventilation; Q̇c: capillary perfusion; Pa: arterial partial pressure; PET: end-tidal partial pressure; Hypervent: alveolar hyperventilation; CIC: end-inspiratory lung volume/total lung capacity≥ 0.9, tidal volume (VT)/inspiratory capacity ≥ 0.7, VT plateau, all reached at an abnormally-low work rate. Ranges of dyspnea severity based on percentiles distribution of scores at a given ventilation as a function of age and sex: 5th–25th: “mild”; 25th–50th: “moderate”; 50th 75th: “severe”; 75th 95th: “very severe” [Citation14]. Reproduced, with permission of the publisher, from: Neder [Citation66].](/cms/asset/499ebab6-5d89-4667-8781-12931df46ad8/icop_a_2301549_f0005_c.jpg)
Figure 6. An illustrative example of how respiratory functional imaging (phase-resolved functional lung (PREFUL) 1H magnetic resonance imaging for ventilation and perfusion [Citation109–113] can be combined with computed tomography-based emphysema extension and distribution (parametric response mapping) to provide relevant insights into the genesis of out-of-proportion dyspnea in patients with mild COPD. This 54 years old woman with preserved FEV1 and out-of-proportion decrease in lung diffusing capacity for carbon monoxide showed high ventilation-CO2 output and an increased physiological dead space (VDphys) on cardiopulmonary exercise testing. Despite trivial emphysema (red and pink), note extensive areas of perfusion deficit percent (QDP) in non-emphysematous regions of the lung which were well ventilated (low ventilation deficit percent (VDP)) at rest and during exercise (light green). These findings are in keeping with the notion that impaired pulmonary perfusion beyond expected by emphysema, may increase the wasted ventilation in these patients, contributing to exertional dyspnea.
![Figure 6. An illustrative example of how respiratory functional imaging (phase-resolved functional lung (PREFUL) 1H magnetic resonance imaging for ventilation and perfusion [Citation109–113] can be combined with computed tomography-based emphysema extension and distribution (parametric response mapping) to provide relevant insights into the genesis of out-of-proportion dyspnea in patients with mild COPD. This 54 years old woman with preserved FEV1 and out-of-proportion decrease in lung diffusing capacity for carbon monoxide showed high ventilation-CO2 output and an increased physiological dead space (VDphys) on cardiopulmonary exercise testing. Despite trivial emphysema (red and pink), note extensive areas of perfusion deficit percent (QDP) in non-emphysematous regions of the lung which were well ventilated (low ventilation deficit percent (VDP)) at rest and during exercise (light green). These findings are in keeping with the notion that impaired pulmonary perfusion beyond expected by emphysema, may increase the wasted ventilation in these patients, contributing to exertional dyspnea.](/cms/asset/0300abbc-25a9-425d-9d69-e129e213045b/icop_a_2301549_f0006_c.jpg)
Table 1. A simplified overview of main invasive and noninvasive physiologic measurements potentially sensitive to disturbances in pulmonary gas exchange efficiency in patients with mild COPD.
