Figures & data
Figure 1 N-terminal sequences of human Cx26 genes studied in this work, and confocal and electron microscopy of Cx26M34Adel2-7 mutant. Sequence alignment of first 35 amino acid residues of two Cx26 N-termini, Cx26M34A and Cx26M34Adel2-7, is shown in (a). Both sequences contain the M34A point mutation. (b) Confocal micrograph of a transiently expressed Cx26M34Adel2-7-GFP in HeLa cells. Gap junction plaque formation between the adjacent cells was determined with the GFP fluorescence (arrows). The scale bar corresponds to 10 μ m. Isolated membrane fractions (c), purified channels (d), and 2D crystal (e) of Cx26M34Adel2-7 were negatively stained with uranyl acetate and observed by conventional EM. This deletion mutant appears to form gap junction plaques. Cx26M34Adel2-7 channels are stable in detergent solution and appear as homogeneous doughnut-shaped particles. The orthorhombic crystal lattice of Cx26M34Adel2-7 is the same packing as Cx26M34A 2D crystals (Oshima et al. Citation2007). Scale bars for (c to e) represents 100 nm.
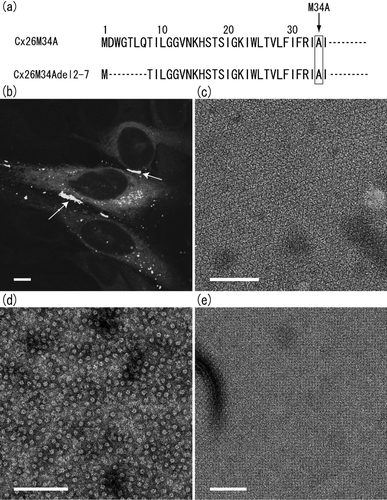
TABLE 1 Summary of electron crystallographic data
Figure 2 Projection maps of Cx26M34A (a) and Cx26M34Adel2-7 (b), and difference map (c) depicted by contours in combination with grey scale. The difference map (c) is drawn by subtraction of Cx26M34Adel2-7 density from Cx26M34A density. The represented area corresponds to one unit cell. The cell dimension of the difference map is consistent with Cx26M34A crystals. Solid and dashed lines represent positive and negative densities, respectively, with 0.2σ step intervals for (a) and (b), 0.5σ step intervals for (c). Asterisks represent the density peaks corresponding to the pore-lining helix C in 3D map (see ).
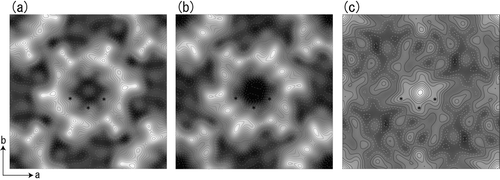
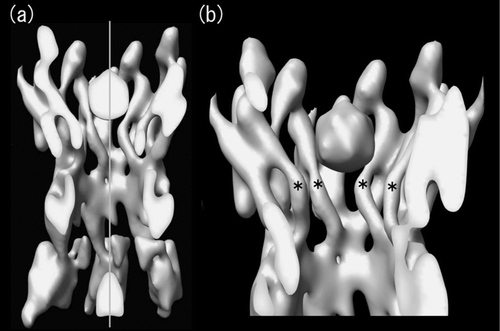
Figure 3 Cross-sectional view of the symmetrized 3D structure of Cx26M34A. Real space non-crystallographic six-fold symmetry has been applied to the original Cx26M34A 3D map (Oshima et al. Citation2007) to increase the signal to noise ratio, thereby enabling better visualization. At this contour level, the tertiary structure of the helices and extracellular domains is well-defined and a plug density is located in each hemichannel. The cross-sectional view of two docked hemichannels through the plugs (a) and the view of a hemichannel containing a whole plug (b) demonstrate that no other density is observed along the axis in the center of the pore. The central pore axis is represented by a perpendicular grey line. The pore-lining helices C are again labelled with asterisks as previously indicated in .