Figures & data
Table 1. Two-hybrid screen identifies human proteins that interact with the unique β1D integrin cytoplasmic domain
Figure 1. (A) Coprecipitation of purified His-CSN3 and GST-β1D on cobalt beads. Lane 1, the eluate from the incubation containing purified GST-β1D fusion protein. Lane 2, the eluate from His-CSN3 fusion protein. Lane 3, the eluate when the incubation contained both His-CSN3 and GST-β1D. The blot was probed with β1D-specific antibody 2B1 (1/200). GST-β1D was only detected (lane 3) when His-CSN3 was present in the incubation. (B) Coprecipitation of purified His-CSN3 and GST-β1D on glutathione-S-transferase–linked beads. Lane 1, the eluate from the incubation containing purified GST-β1D fusion protein. Lane 2, the eluate from His-CSN3 fusion protein. Lane 3, the eluate when the incubation contained both His-CSN3 and GST-β1D. The blot was probed with V5-specific antibody. His/V5-CSN3 was only detected (lane 3) when GST-β1D was present in the incubation. (C) Immunoprecipitation of both β1D integrin and CSN5- with CSN3-specific antibody (1/2000; Bethyl Labs, BL564). Lane 1, total cell lysate. Lane 2, the IP eluate. The blots were probed with β1D (2B1) and CSN5 (1/1000; AB495, ABcam) antibodies. (D) The immunobolt shown in C probed with an antibody specific to β1A integrin (MC229).
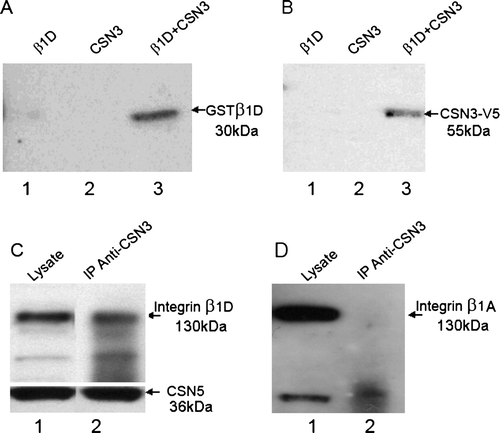
Figure 2. Immunofluorescence microscopy of adult cardiac myocytes and proliferating myoblasts. (A) CSN3 (green) was detected with PW 8235 (Biomol), α-actinin (red) with EA-53 (Sigma), and the nucleus with bis-benzamide (blue). Orange/yellow shows colocalization of some CSN3 with α-actinin at the Z-bands. The inset shows a similar pattern of localization for β1D integrin (9EG7). (B) CSN3 (green) localized in a punctate pattern in both the cytoplasm and nucleus of proliferating C2C12 myoblasts. However, it does not localize with β1A integrins (red) at focal adhesions.
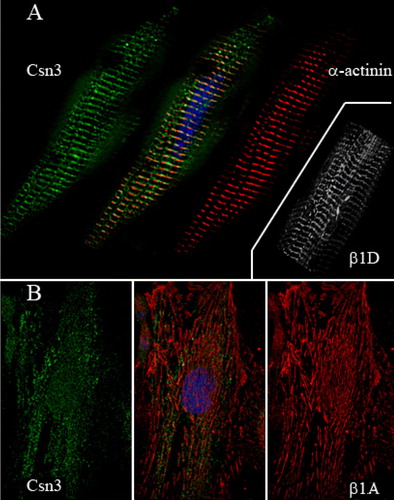
Figure 3. Representative fractionation and immunoblot analysis of the COP9 signalosome subunits in C2C12 skeletal myoblasts/tubes. Proliferating (P) C2C12 myoblast fractions. C2C12 myoblast/tube fractions after 10 days (D10) of differentiation. C2C12 myotube fractions after 14 days (D14) of differentiation. Four fractions were examined: cytoplasm, mitochondrial, nuclear, and membrane. Antibodies specific to various COP9 subunits are indicated. Note: CSN3 was only found with the membrane fraction in differentiated C2C12 cells when β1D integrin was expressed (data not shown).
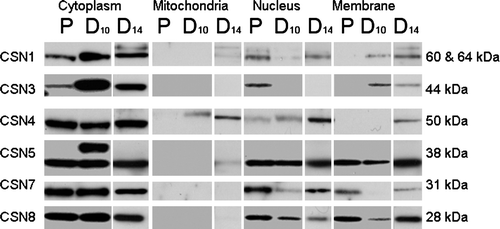
Figure 4. Fractionation and immunoblot analysis of the COP9 signalosome subunits in heart. Four adult mouse hearts and two pools of embryonic hearts (E16/17; 20 hearts examined in duplicate) were isolated and fractionated into soluble cytosol, mitochondria (5000×g), nuclear (600×g), and microsomal (membrane 100,000×g) fractions. Fractions were analyzed by immunoblot analysis with subunit-specific antibodies as indicated. Resultant blots were analyzed with Un-Scanit (Silk Scientific). Results show the mean distribution of all experiments ± SEM. (A) Histogram illustrating the amount (percentage of total) of CSN3 and CSN5 in embryonic fractions and the change in distribution in adult hearts. Notice the large shift of CSN3 from the cytosol to the nucleus of adult lysates. (B) Histogram showing the distribution of CSN1, CSN4, CSN7, and CSN8 in embryonic hearts. (C) Histogram showing the distribution of CSN1, CSN4, CSN7, and CSN8 in adult hearts. Note: β1D integrin is predominantly found in the membrane fractions of adult hearts (data not shown).
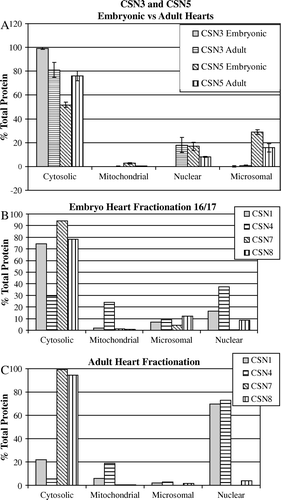
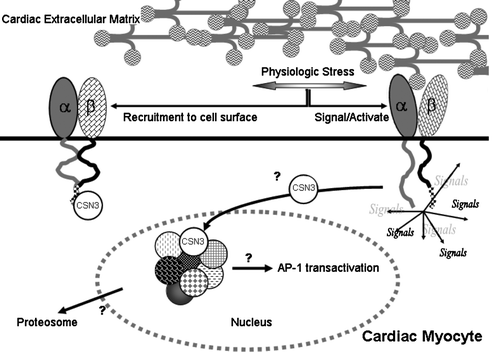
Figure 5. Model of β1D integrin interaction with the COP9 signalosome complex. Cardiac myocytes respond to physiological stress (e.g., ischemia, pressure or volume fluctuations) through integrin activation. The resultant signaling causes translocation of CSN3-containing (sub)complexes to the nucleus, recruitment of more integrin to the cell surface, and strengthening of cell-ECM linkages. The recruitment of CSN3 to the nucleus results in the regulation of various transcripts responsive to the COP9 signalosome (sub)complex and the selective export of proteins from the nucleus for degradation pathways.