Figures & data
Figure 1. Adult mouse cardiac fibroblast cultures express vimentin (A) and DDR2 (B). A subset of cells also expresses α-SMA (C). Cx43 signal is punctate and diffuse in both adult (E) and neonatal mouse fibroblasts (G). Neonatal rat myocytes exhibit the typical cardiac Cx43 staining at intercellular junctions (I). Pan-cadherin staining is evident at cell-cell junctions in each cell type (D, F, H). The limited appositional membrane staining in adult cardiac fibroblasts belies the fact that the fibroblast cultures are well coupled. Bar = 50 µm.
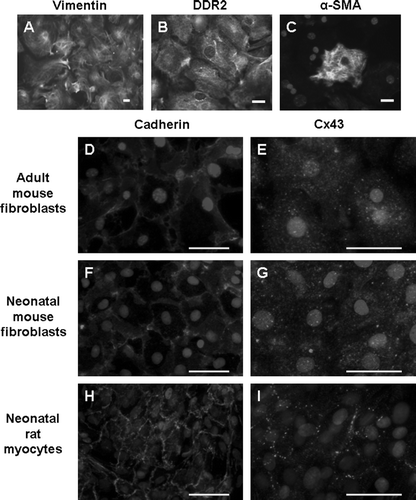
Figure 2. Adult mouse cardiac fibroblasts express both Cx43 and Cx45. (A) The first four lanes represent adult myocyte extracts. The last four lanes represent adult fibroblast extracts. Equal protein (30 µg) was loaded in each lane. Fibroblasts express less Cx43 than myocytes. Cx45 is comparable in fibroblasts and myocytes, but Cx45 in fibroblasts migrated faster than that in myocytes. (B) Relative expression of Cx43 and Cx45 in adult cardiac myocytes and fibroblasts. The ratio of Cx45 to Cx43 band density obtained from myocytes isolated from five different hearts and fibroblasts obtained from seven different hearts is significantly larger for fibroblasts than for myocytes.
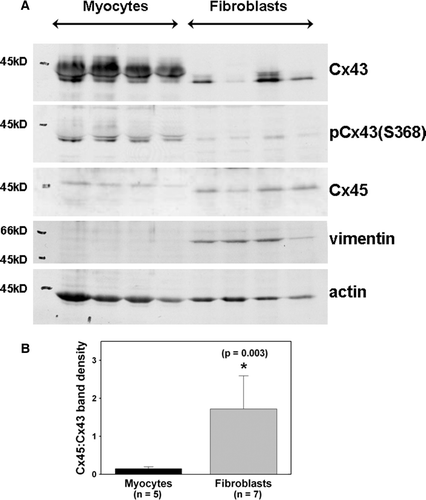
Figure 3. Alkaline phosphatase treatment (+) of myocyte and fibroblast extracts demonstrates that dephosphorylation results in migration of a single band at 41 kDa → in each cell type. The top and center images are different exposures of the same membrane blotted with anti-Cx43 antibody. Two exposures are shown to illustrate alkaline phosphatase treatment in myocytes (top) and fibroblasts (center) more clearly. The bottom image shows the same membrane blotted with anti-Cx45 antiserum. Equal amount of protein (30 µg) was loaded in each lane.
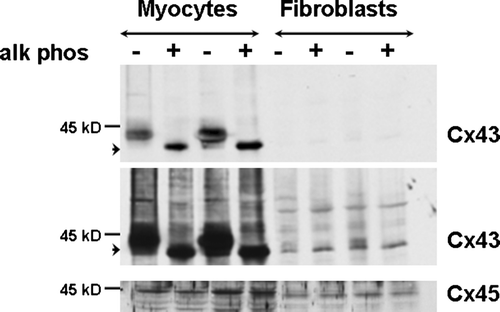
Figure 4. Cx43 expression and intercellular coupling are reduced in Cx43-deficient cardiac fibroblasts. (A) Cx43 expression (top) is reduced in cardiac fibroblasts isolated from Cx43+/ − hearts (+/ − ) compared to wild-type (+/ + ), as shown in this representative immunoblot. Cx45 expression (bottom) is not altered as shown in a reprobe of the same immunoblot. Equal amount of protein (30 µg) was loaded in each lane. (B) Histograms of summarized data from fibroblasts isolated from eight wild-type (Cx43 WT) and seven Cx43+/ − (Cx43 HT) hearts demonstrating that Cx43 expression was significantly reduced by ∼50% in Cx43+/ − fibroblasts. (C) Fluorescence images showing Lucifer yellow dye transfer after scrape-loading in wild-type (left) and Cx43+/ − (Cx43 HT; right) adult murine cardiac fibroblast cultures. Outlines beside each fluorescence image represent the areas of dye transfer obtained using ImageJ software. The length of the microscopic field analyzed is shown by the double-headed arrow. The total area of dye transfer was divided by this length to calculate the distance of dye transfer in µm. (D) Histograms of summarized data showing a 24% reduction in distance of dye transfer in Cx43-deficient fibroblasts.
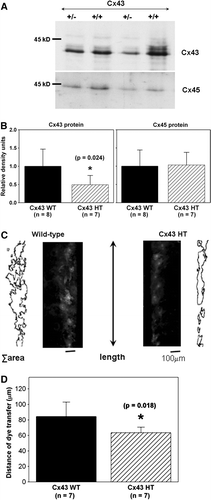
Figure 5. AdVCx43 infection resulted in overexpression of Cx43 and increased intercellular coupling in adult cardiac fibroblasts. (A) Fibroblasts isolated from wild-type hearts treated with control adenoviral (Cont; first lane) or AdVCx43 (last three lanes) constructs (MOI 50 to 100), blotted with anti-Cx43 antibody (top). The same membrane was blotted with anti-vimentin antibody (bottom). Equal amount of protein (20 µg) was loaded in each lane and Cx43 band densities were normalized to vimentin band densities. Each value was then normalized to the control values on each membrane. (B) Histograms of summarized data showing a 1.6-fold increase in Cx43 protein expression in AdVCx43-infected fibroblasts. (C) Fluorescence images showing Lucifer yellow dye transfer after scrape-loading in wild-type control adenovirus-infected (Control AdV; MOI 100; left) and AdVCx43-infected (MOI 100; right) adult murine cardiac fibroblast cultures. Outlines beside each fluorescence image represent the areas of dye transfer obtained using ImageJ software. The distance of dye transfer was calculated as described in the legend for and in the text. (D) Histograms of summarized data showing a 60% increase in distance of dye transfer in fibroblasts overexpressing Cx43.
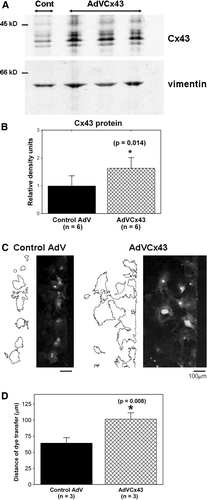
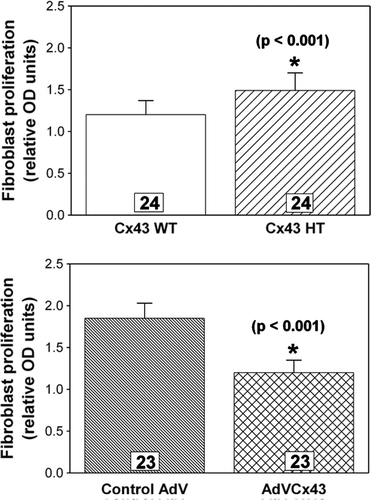
Figure 6. Fibroblast proliferation measured by a BrdU colorimetric ELISA assay is inversely related to the level of Cx43 expression. Cx43-deficient fibroblasts (Cx43 HT) exhibit significantly (p<0.001) greater proliferation (top), whereas Cx43-overexpressing fibroblasts (AdVCx43) exhibit significantly (p<0.001) less proliferation (bottom) compared to wild-type (Cx43 WT) or control adenovirus (AdV)-infected cultures, respectively. n's are individually plated wells from four independent hearts for each genotype or treatment.