Figures & data
Figure 1. N-cadherin expression decreases as the endothelial monolayer matures. (A) Human dermal microvascular endothelial cells (HDMECs) were plated at a density of 8.0×104 cells/cm2, and phase contrast images were taken every 24 h to document degree of confluence. Bar = 50 µm. (B) Top: Cells were lysed at each time point, and proteins were separated by SDS-PAGE. Following transfer to nitrocellulose membranes, lysates were immunoblotted for VE-cadherin, N-cadherin, and β-actin as a loading control. Bottom: Densitometric analysis of immunoblots. Values are normalized to β-actin and are expressed relative to the 24-h time point (mean±SEM; n=6). N-cadherin: p=.0011 by one-way ANOVA. (C) Localization of VE-cadherin and N-cadherin in HDMECs during growth to confluence Cells plated at 8.0×104 cells/cm2 were fixed at the 24- or 96-h time points, and immunofluorescence microscopy was performed by incubating with antibodies to either VE-cadherin (left panels) or N-cadherin (right panels) followed by fluorescent-conjugated secondary antibodies. Bar = 50 µm. (D) RNA was isolated at each time point, and quantitative real-time PCR was performed using primers to VE-cadherin, N-cadherin, and GAPDH, and copy number was determined using standard curves (Leong et al. Citation2007). Values for each time point are expressed relative to the 24-h time point, and are normalized to GAPDH (mean±SEM; n=6). N-cadherin mRNA: p = .0167 by one-way ANOVA.
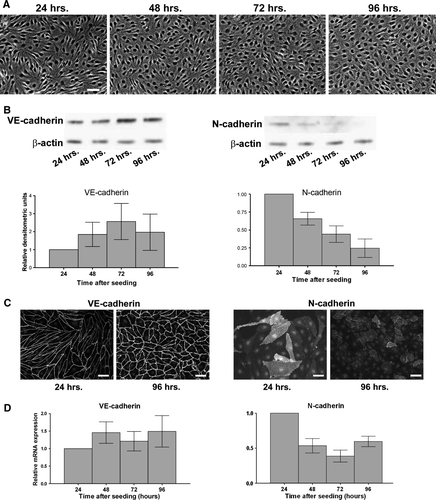
Figure 2. VE-cadherin levels in HUVECs are maintained in the absence of N-cadherin. Top panels: siRNA sequences targeting either luciferase (lucif. siRNA) or N-cadherin (Nsmrt, N6D, or N25mod) were delivered into HUVECs via electroporation, and cells were plated at confluence. Immunoblot analysis for N-cadherin (left), VE-cadherin (right), and β-actin was carried out 48 h after plating. Densitometric analysis of immunoblots is shown below. Values for N-cadherin and VE-cadherin are normalized to β-actin and are expressed relative to luciferase control (mean±SEM; minimum n=3, for N6D p<.0001, N25mod p=.0024, Nsmrt p<.0001). N6D: individual chemically modified sequence purchased from Dharmacon. Nsmrt: N-cadherin siGENOME Smartpools (unmodified) purchased from Dharmacon. N25mod: individual sequence purchased from Ambion, subsequently chemically modified by Dharmacon.
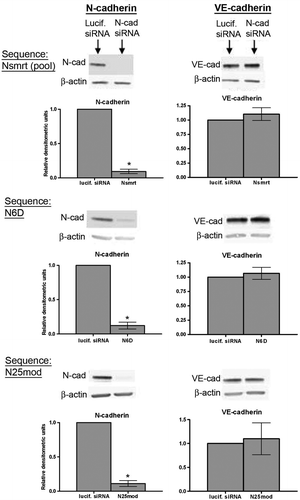
Figure 3. N-cadherin protein expression varies in endothelial cells from different sources. (A) Confluent monolayers of HLMECs, HUVECs, HPAECs, and BPAECs were lysed 72 to 96 h after plating, and proteins were separated by SDS-PAGE. Following transfer to nitrocellulose membranes, lysates were immunoblotted for VE-cadherin (left) and N-cadherin (right). β-Actin was used as a loading control. (B) Densitometric analysis of immunoblots shown in A. Densitometric values were normalized to β-actin and values are expressed relative to BPAECs (SEM; n = 3).
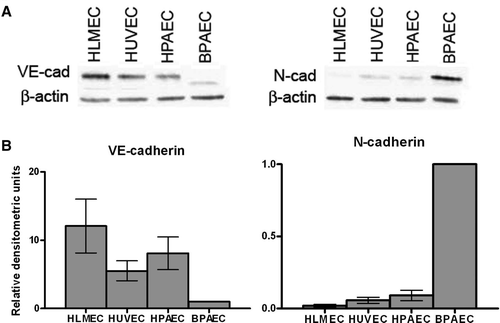
Figure 4. Relationship between VE-cadherin and N-cadherin protein levels in BPAECs. (A) siRNA sequences targeting either luciferase or N-cadherin were delivered into BPAECs via electroporation, and cells were plated at confluence. Immunoblot analysis for N-cadherin, VE-cadherin, and β-actin was carried out 48 h after plating. Densitometric analysis: Values for N-cadherin and VE-cadherin are normalized to β-actin and are expressed relative to luciferase control (mean±SEM, n=8; for N-cad p<.0001, VE-cad p=.0321 using single-sample t test. N siRNA: Individual sequence targeting bovine N-cadherin purchased from Ambion.) *Significance (p<.05) as determined using single-sample t test. (B) Confluent BPAEC monolayers were infected with either adenovirus containing GFP or one of two doses of flag epitope–tagged N-cadherin (AdN-cad-flag). After 48 h, cells were lysed, and N-cadherin and VE-cadherin were detected in lysates by immunoblot analysis. β-Actin was used as a loading control (bottom panels). (C) Top: Confluent BPAEC monolayers were infected with either GFP or one of three doses of VE-cadherin-flag adenovirus (AdVE-cad-flag) and immunoblotted for N-cadherin and VE-cadherin. Right, Densitometric analysis: Densitometric values for cadherins are normalized to β-actin expressed relative to control GFP infection (mean±SEM, n=3; for AdN-cad-flag, VE-cad p = .0197 by one-way ANOVA; for AdVE-cad-flag, N-cad p<.0001 by one-way ANOVA). Bottom: Following the 48-h infection with AdVE-cad-flag, immunofluorescence microscopy was performed on BPAECs using an antibody to N-cadherin, followed by a fluorescent-conjugated secondary antibody. Note the decrease in N-cadherin staining intensity with increasing dose of AdVE-cad-flag (left to right). Bar = 50 µm.
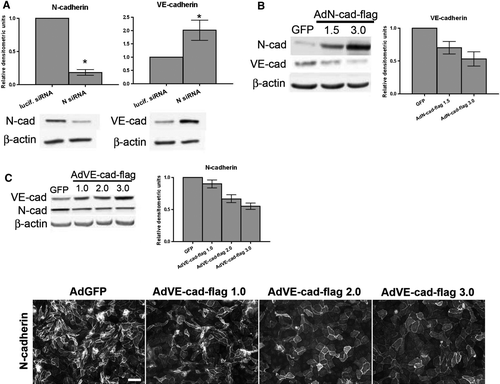
Figure 5. Regulation of cadherin levels by p120. (A) siRNA targeted to either luciferase (lucif. siRNA) or p120 was delivered via electroporation into BPAECs. After 48 h, cells were lysed and immunoblot analysis was carried out for p120 (left), VE-cadherin (center), and N-cadherin (right). β-Actin was used as a loading control (not shown). Densitometric values are normalized to β-actin and are expressed relative to luciferase siRNA (mean ± SEM, n=3; for p120 p=.0043, VE-cad p = .0199, N-cad p = .0166). (B) Confluent monolayers of BPAECs were infected with either GFP adenovirus or p120-GFP adenovirus. Forty-eight hours after infection, cells were lysed and immunoblot analysis was carried out for p120, VE-cadherin, N-cadherin, and β-actin as loading control. Densitometric values (right) for cadherins are normalized to β-actin and are expressed relative to GFP infection (mean ± SEM, n=5; for VE-cadherin p=.0132). *Significance (p<.05) as determined using single-sample t test. (C) Similar proportions of VE-cadherin and N-cadherin associate with p120 under control conditions. Lysates of BPAECs were subject to two consecutive cycles of immunoprecipitation for p120. Left: Immunoblot of each p120 immunoprecipitate for VE-cadherin (left lanes) and N-cadherin (right lanes). Center: Samples taken from lysate before the first immunoprecipitation (Pre-IP) and from supernatants following each immunoprecipitation (Post-IP (1) and (2)) were immunoblotted for VE-cadherin, N-cadherin, p120, and GAPDH. Right: graph represents percent of total VE-cadherin and N-cadherin precipitated from lysate in each p120 immunoprecipitation step, as analyzed by densitometry. Densitometric values for each cadherin were normalized to GAPDH to account for any dilution of the lysate and are displayed as percent of total (mean ± SEM, n=4). (D) BPAEC monolayers were infected with adenovirus containing either GFP (AdGFP), the extracellular and transmembrane portions of the IL-2 receptor fused to the cytoplasmic domain of VE-cadherin with a Myc-epitope tag (AdIL-2R-VE-cadcyto), or the extracellular and transmembrane portions of the IL-2 receptor fused to a p120-uncoupled cytoplasmic domain of VE-cadherin with a Myc-epitope tag (AdIL-2R-VE-cadAAA). 48 h after infection, cells were lysed and immunoblot analysis was carried out for N-cadherin, the Myc-epitope tag to detect the fusion proteins, and β-actin as a loading control. Blots representative of four separate experiments.
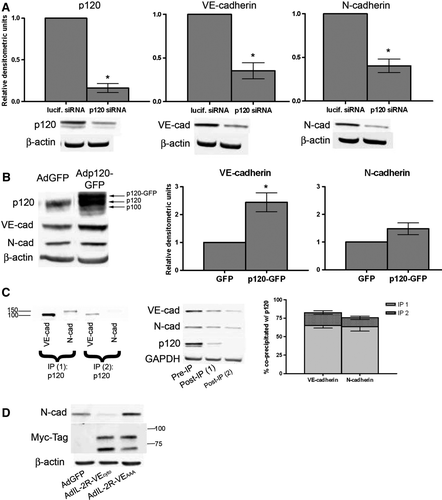
Figure 6. Modulation of N-cadherin mRNA expression by mural cells, serum treatment. (A) Coculture systems (Fillinger et al. Citation1997): Top: Direct contact. Rat vascular smooth muscle cells (VSMCs) were seeded on the bottom surface of a 0.4-µm pore Transwell cell culture insert. Twenty-four hours later, HDMECs were seeded on the top side of the wells, and were allowed to grow for 96 h. Bottom: No contact. VSMCs were seeded on 100-mm plate. Twenty-four hours later, HDMECs were seeded on a 0.4-µm pore Transwell cell culture insert that was placed in the 100-mm plate, and were allowed to grow for 96 h. HDMECs (not shown): cells were seeded on a 0.4-µm pore Transwell cell culture insert that was placed in an empty 100-mm plate and were allowed to grow for 96 h. (B) RNA was isolated from HDMECs. Graphs show VE-cadherin and N-cadherin mRNA expression. Values are normalized to GAPDH, and are expressed relative to HDMECs (mean±SEM, n=4; *significance [p<.05] as determined using single-sample t test; for N-cad no contact p = .0068, N-cad contact p = .0010). (C) HDMECs were seeded at confluence in growth medium. After 72 h medium was changed to either basal medium (EBM) or basal medium supplemented with 5% FBS (5%). After 24 h RNA was isolated. Graphs show VE-cadherin and N-cadherin mRNA expression. Values are normalized to GAPDH and are expressed relative to EBM-treated cells (mean±SEM, n=3; *significance [p<.05] as determined using single-sample t test; for N-cad p = .0182).
![Figure 6. Modulation of N-cadherin mRNA expression by mural cells, serum treatment. (A) Coculture systems (Fillinger et al. Citation1997): Top: Direct contact. Rat vascular smooth muscle cells (VSMCs) were seeded on the bottom surface of a 0.4-µm pore Transwell cell culture insert. Twenty-four hours later, HDMECs were seeded on the top side of the wells, and were allowed to grow for 96 h. Bottom: No contact. VSMCs were seeded on 100-mm plate. Twenty-four hours later, HDMECs were seeded on a 0.4-µm pore Transwell cell culture insert that was placed in the 100-mm plate, and were allowed to grow for 96 h. HDMECs (not shown): cells were seeded on a 0.4-µm pore Transwell cell culture insert that was placed in an empty 100-mm plate and were allowed to grow for 96 h. (B) RNA was isolated from HDMECs. Graphs show VE-cadherin and N-cadherin mRNA expression. Values are normalized to GAPDH, and are expressed relative to HDMECs (mean±SEM, n=4; *significance [p<.05] as determined using single-sample t test; for N-cad no contact p = .0068, N-cad contact p = .0010). (C) HDMECs were seeded at confluence in growth medium. After 72 h medium was changed to either basal medium (EBM) or basal medium supplemented with 5% FBS (5%). After 24 h RNA was isolated. Graphs show VE-cadherin and N-cadherin mRNA expression. Values are normalized to GAPDH and are expressed relative to EBM-treated cells (mean±SEM, n=3; *significance [p<.05] as determined using single-sample t test; for N-cad p = .0182).](/cms/asset/ab2077f7-3bca-44fe-85ef-a3d21b2bd2fc/icac_a_344205_f0006_b.gif)