Figures & data
Figure 1. Relative positions and lengths of the various truncation mutants of connexin43 used in this study. Numbers refer to amino acid position in the connexin43 protein sequence. CLFL, full-length sequence of the connexin43 cytoplasmic loop; CTFL, full-length sequence of the connexin43 carboxy-terminus; CTTr, truncated connexin43 carboxy-terminus sequence.
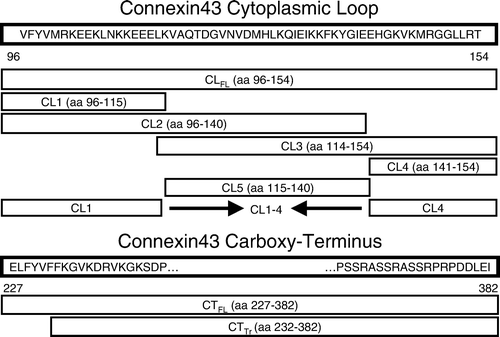
Figure 2. Coomassie staining of protein samples obtained from glutathione-S-transferase (GST) pull-down assays. Products used in the assay included the full-length cytoplasmic loop (CLFL) sequence of Cx43 fused to GST and GST alone. These products were allowed to bind glutathione sepharose beads and were then eluted and collected (right-most two lanes) or exposed to mouse brain lysate prior to elution (left-most two lanes). Excess protein bands in the left-most lane containing eluate from the GST-CLFL beads exposed to brain lysate suggest novel proteomic interactions involving the Cx43-CLFL.
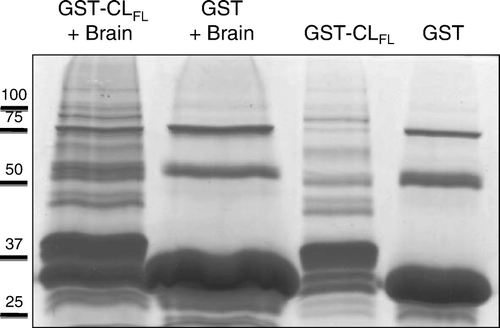
Figure 3. Two-dimensional gel electrophoresis of GST pull-down assay involving Cx43-CLFL and matched controls. GST pull down assay with Cx43-CLFL GST fusion product followed by 2D gel electrophoresis (a) reveals five spots not seen in control experiments (b and c).
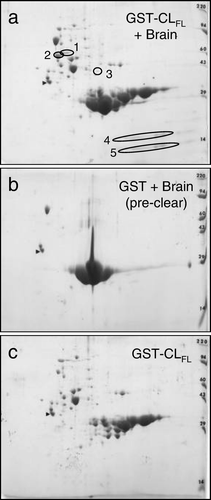
Figure 4. Mass spectrometry (MS) data acquired from analyzing spot 2 in Figure 3a. Identified peptide sequences (examples as shown) are consistent with β-tubulin.
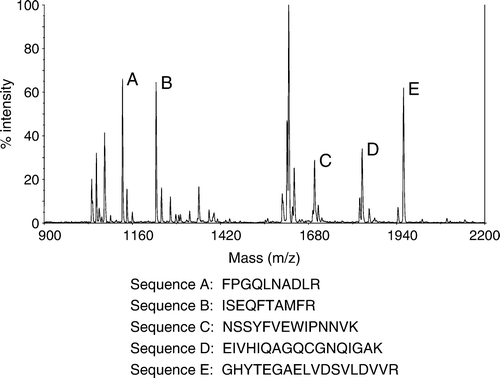
Table 1. Proteins identified by 2-D gel electrophoresis and mass spectrometry
Figure 5. Comparisons of relative affinity for β-tubulin between Cx43-CLFL, portions of the CL of Cx43 and the carboxy terminus of Cx43 (Cx43-CTFL). (a) β-Tubulin abundance detected by Western blotting is shown in eluates from GST pull-down assays using GST fusions with Cx43-CLFL and truncated CL products, CL1-4 and CL5. Minimal β-tubulin is detected in the assay involving CL1-4 and no appreciable β-tubulin is seen in the eluate from the CL5 assay. (b) β-Tubulin abundance is substantially greater in eluates from assays involving the Cx43-CLFL GST fusion product than those involving either CL2 or CL3. (c) Eluates from GST pull down assays involving Cx43-CTFL contain more β-tubulin than those involving Cx43-CLFL. Shown also is an example of an eluate from an assay involving the GST construct without an insert. This control was performed for all of the experiments and demonstrated no tubulin signal. (d) Cx43-CTFL (lane 1) binds β-tubulin with fivefold greater affinity than a construct containing the sequence from the cytoplasmic tail of Cx43 in which the first five amino acids have been removed (CTTr) (lane 3). Lane 3 has 5X more protein loaded than lane 1.
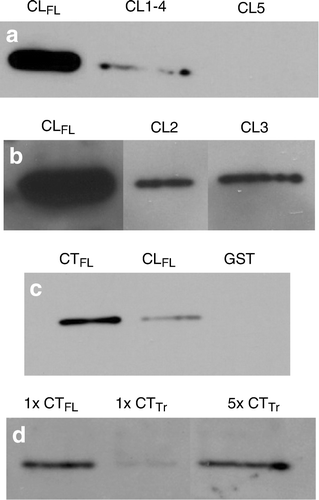
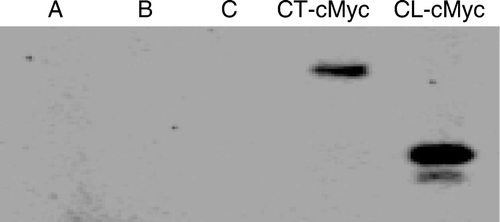
Figure 6. Immunoprecipitation of Cx43-CT-cMyc and Cx43-CL-cMyc fusion products with anti-β-tubulin antibodies. Polyacrylamide gel electrophoresis was performed to separate proteins, followed by probing with anti-cMyc antibodies. Controls are shown in lanes A (PCS2 vector–transfected HeLa cell lysate, immunoprecipitated with anti-β-tubulin), B (PCS2 vector containing CT-cMyc-transfected HeLa cell lysate, not exposed to anti-β-tubulin), and C (PCS2 vector–containing CL-cMyc-transfected HeLa cell lysate, not exposed to anti-β-tubulin).