Figures & data
Figure 2. Effect of the adsorption bath pH on adsorption quantity of RB 19 onto EGHP (Conditions of steam explosion: 1.2 MPa, 120 s; Adsorption conditions: initial dye concentration 3000 mg/L, pH 2.0–9.5, amount of EGHP 10 g/L.).
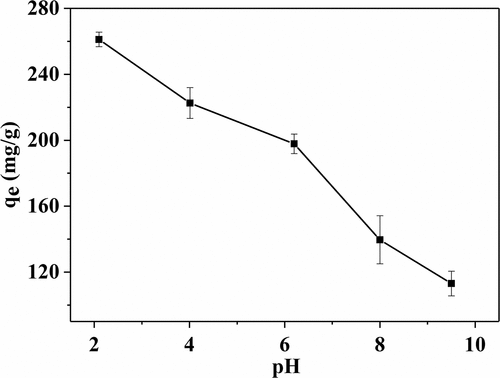
Figure 3. Adsorption mechanisms of RB 19 on at pH value lower than its isoelectric point of around pH 4.1.
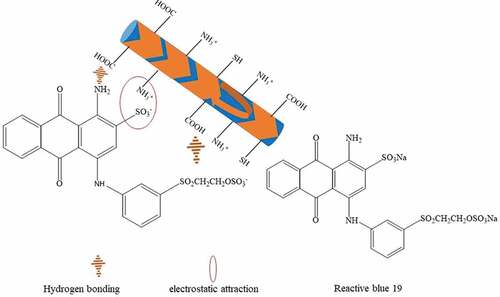
Figure 4. (a) Adsorption kinetic curves and fitted adsorption kinetic curves of RB 19 onto CGH, FDGH and EGHP with (b) pseudo-first-order and (c) pseudo-second-order model (Conditions of steam explosion: 1.3 MPa, 150 s; Adsorption conditions: pH 2.0, initial dye concentration for CGH 300 mg/L, for FDGH 1400 mg/L and for EGHP 3000 mg/L, amount of CGH, FDGH and EGHP 10 g/L.).
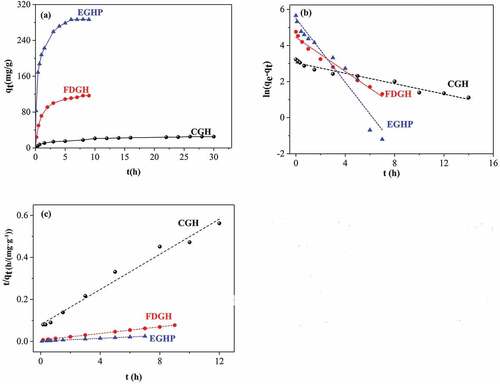
Table 1. Adsorption kinetic parameters of RB19 onto CGH, FDGH and EGHP.
Figure 5. Removal efficiency and adsorption at equilibrium of (a) CGH, (b) FDGH and (c) EGHP toward RB19 under the different initial dye concentration; Langmuir and Freundlich adsorption isotherm curves of (d) CGH, (e) FDGH and (f) EGHP toward RB19.(Adsorption conditions for CGH: initial dye concentration 50–1100 mg/L, pH 2.0, amount of CGH 10 g/L, adsorption time 28 h; Adsorption conditions for FDGH: initial dye concentration 400–3000 mg/L, pH 2.0, amount of FDGH 10 g/L, adsorption time 8 h; Conditions of steam explosion: 1.3 MPa,150 s; Adsorption conditions for EGHP: initial dye concentration 2400–5000 mg/L, pH 2.0, amount of EGHP 10 g/L, adsorption time 8 h.).
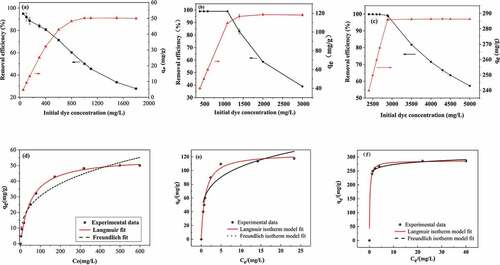
Table 2. Adsorption isotherm parameters and correlation coefficient R2 of fitting the adsorption of RB19 on goat hair with Langmuir and Freundlich isotherm models.
Figure 6. Effects of (a) steam pressure and (b) steam treatment time on adsorption capacity of EGHP toward RB19(Adsorption conditions: initial dye concentration 3500 mg/L, pH 2.0, amount of EGHP 10 g/L, adsorption time 8 h.).
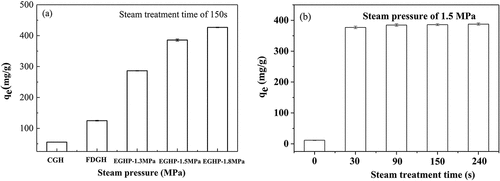
Figure 7. SEM images (х800) of goat hair (a) before and (b) ~ (h) after being steam exploded under different conditions.
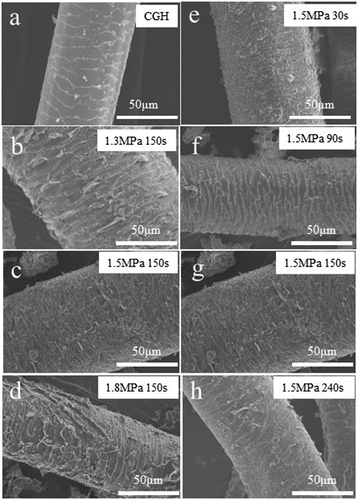
Figure 9. XRD spectra of CGH and EGHP after the different steam explosion conditions (a) Different steam pressure for the steam treatment time of 150 s; (b) the steam pressure of 1.5 MPa for different time of 30–240 s.
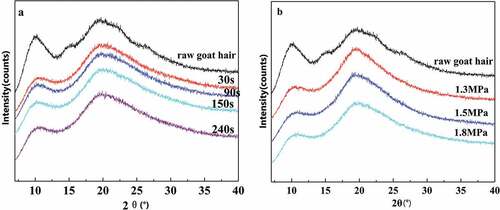
Table 3. Effect of steam pressure and steam treatment time on the crystal index (CI) of EGHP.
Figure 10. Nitrogen adsorption-desorption isotherm curves for different goat hair (Lower curve: adsorption curve; Upper curve: desorption curve). (a) CGH, (b) FDGH, (c) EGHP-1.3mpa, (d)eghp-1.5mpa and (e)eghp-1.8mpa.
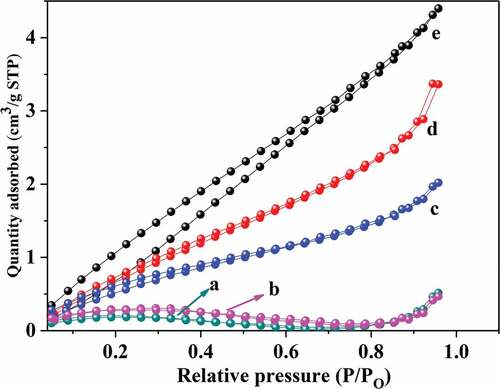
Table 4. BET surface area and pore size of CGH, FDGH and EGHP.
Table 5. Concentration of free NH2 groups for CGH, FDGH and EGHP.
Figure 11. The adsorption capacity of EGHP after different adsorption-desorption cycles toward RB 19 (Conditions of steaming explosion: 1.3 MPa, 150 s; Adsorption conditions: initial dye concentration 3500 mg/L, pH 2.0, amount of EGHP 10 g/L).
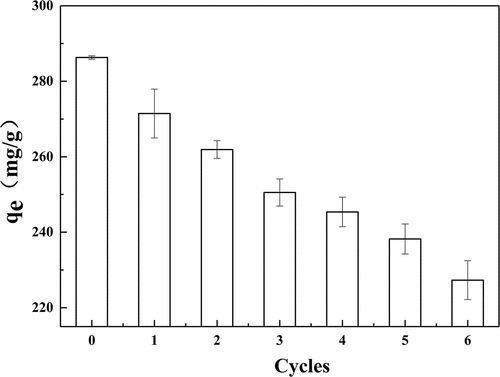
Table 6. Comparison of adsorption capacities of other recently reported adsorbents for the anionic dyes.


