Figures & data
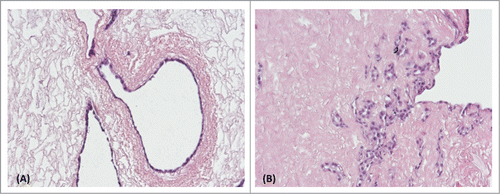
Figure 1. Native (A-1) and decellularized (B-1) porcine kidneys. Cells are visible in the native renal ECM (A-2). H&E staining confirmed complete cell removal from the decellularized ECM (B-2).
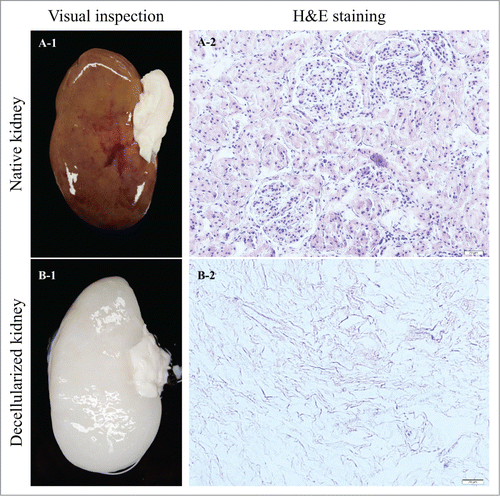
Figure 2. Stress/strain curves representing the upper and lower bounds for frozen/thawed and non-frozen samples (n = 20). Freezing/thawing significantly reduced the modulus of elasticity of native kidneys (P value < 0.0001); however, there was not an additive effect of freezing/thawing for decellularized renal ECM (P value = 0.0636).
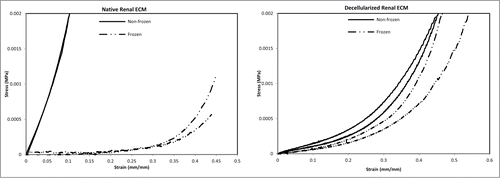
Figure 3. Elastic moduli for frozen/thawed and non-frozen samples (n = 15-22 per group) were calculated as the slope of the linear part of the stress/strain curves at low strains. Freezing/thawing caused a significant reduction in elastic modulus of native kidneys (NK), while no significant reduction was observed for decellularized kidneys (DK). Data are reported in log-transformed format for a more clear comparison and reliable statistical analysis.
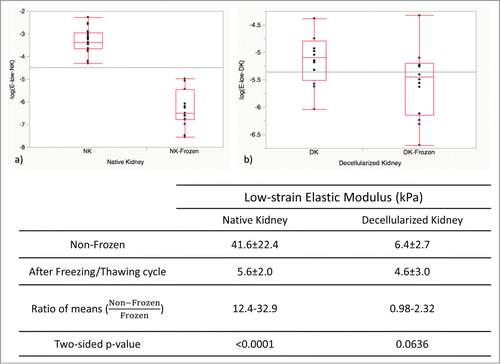
Figure 4. Arterial pressure measurements in frozen/thawed and non-frozen native kidneys (NK) and decellularized kidneys (DK) (n = 12) were indicative of renal vasculature integrity. Arterial pressure was highly reduced for NK after freezing/thawing (P < 0.0001), while it was essentially unchanged for DK (p = 0.18).
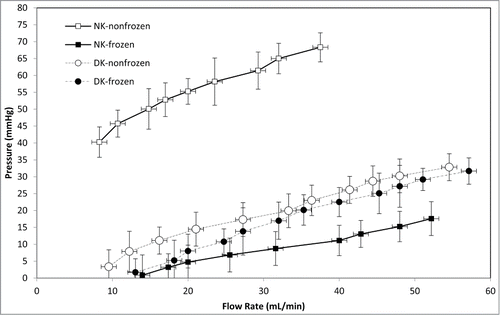
Figure 5. Scanning electron microscopy images of frozen/thawed and non-frozen kidney samples at 250X (A1-D1) and 500X (A2-D2) magnifications. No microstructural damage was detected after freezing/thawing. Renal corpuscles, distal and proximal tubules all were preserved during freezing/thawing cycles.
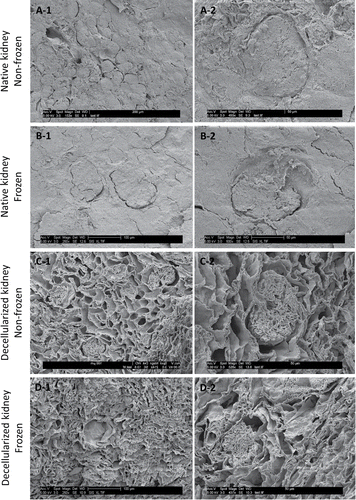
Figure 6. Orcein stain imaging for frozen/thawed and non-frozen native kidney samples at magnifications of 10X (A-1 & B-1) and 20X (A-2 & B-2). Generally, the elastin fibers were damaged as a result of freezing/thawing (affinity for Orcein stain color was lower for frozen/thawed samples which resulted in lighter color in images). Because of fibril damage the structure was more porous and had less integrity as indicated by the white spaces that were more frequent in frozen/thawed samples (arrows show renal corpuscles).
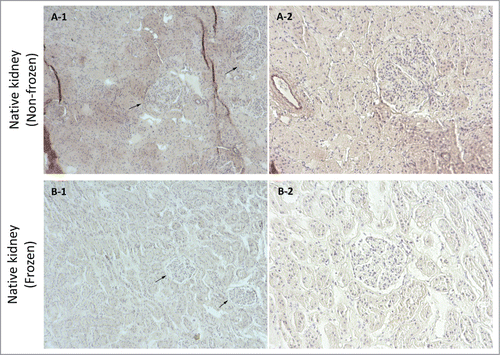
Figure 7. Orcein stain imaging for frozen/thawed and non-frozen decellularized kidney samples at magnifications of 10X (A-1 & B-1) and 20X (A-2 & B-2). Generally, the elastin fibers were damaged as a result of freezing/thawing (affinity for Orcein stain color was lower for frozen/thawed samples which resulted in lighter color in images). Also it appeared that because of fibril damage the structure was more porous and had less integrity as white spaces were more frequent in frozen/thawed samples (arrows show renal corpuscles).
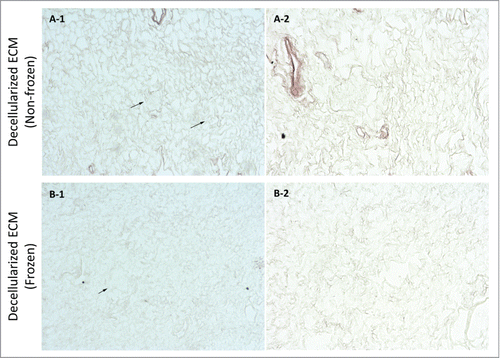
Figure 8. Sirius red stain imaging representative of general collagen for frozen/thawed and non-frozen native kidney samples at 10X (A-1 & B-1) and 20X (A-2 & B-2) magnifications. General collagen appeared to be partially damaged through freezing/thawing as evidenced by decreased stain color in frozen/thawed samples, which was detected as lighter colors. Void spaces were more detectable in frozen/thawed samples, which was again indicative of fibril damage and less integrity (arrows show renal corpuscles).
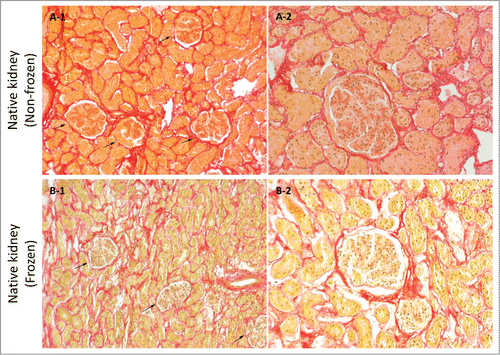
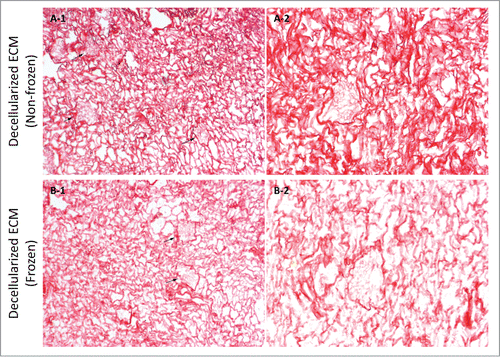
Figure 9. Sirius red stain imaging representative of general collagen for frozen/thawed and non-frozen decellularized kidney samples at 10X (A-1 & B-1) and 20X (A-2 & B-2) magnifications. General collagen seemed to be partially damaged through freezing/thawing since stain color was lighter for frozen/thawed samples; however, the difference was not as stark as for native kidneys after freezing/thawing. Also, the porosity was greater in frozen/thawed decellularized kidney samples, which is again indicative of fibril damage and diminished integrity (arrows show renal corpuscles).