Figures & data
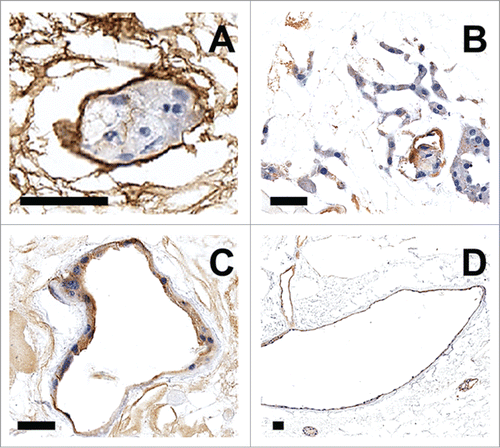
Figure 1. Pig kidneys pre- and post-decellularization and collagen-IV staining of glomerular structures. Gross image of pig kidney pre- (A) and post-decellularization (B), scale bar 1 cm. Collagen-IV immunohistochemical staining of basement membranes (C–H). Pig kidney histology before decellularization (C–E) and after (F–H); arrow heads point to acellular glomerular vascular tufts (G,H). Scale bar (C–H) 100 μm.
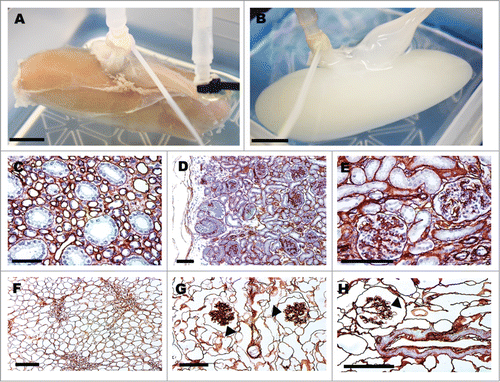
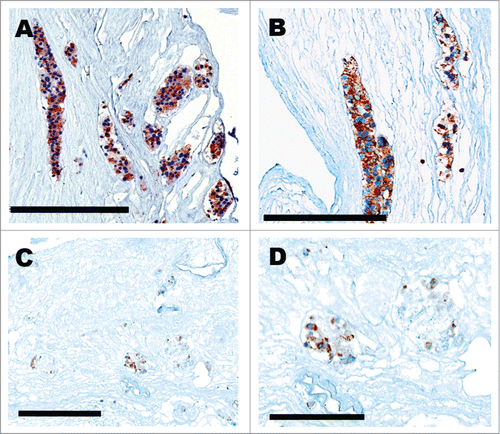
Figure 2. Fluorescent 10 μm polystyrene microspheres (FluoSpheres®) with antegrade perfusion in renal artery (red), (A) visualizing afferent arteriole and glomerulus and retrograde perfusion in ureter (green), (B). Scale bar (A, B) 1 cm. Ten μm polystyrene FluoSpheres® (arrowhead) injected retrograde in the ureter and reaching Bowman's space. Scale bar (C, D) 50 μm.
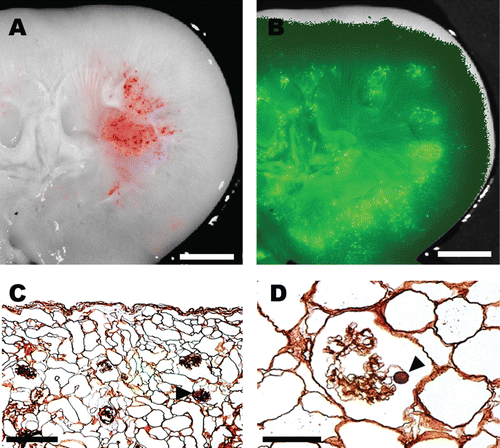
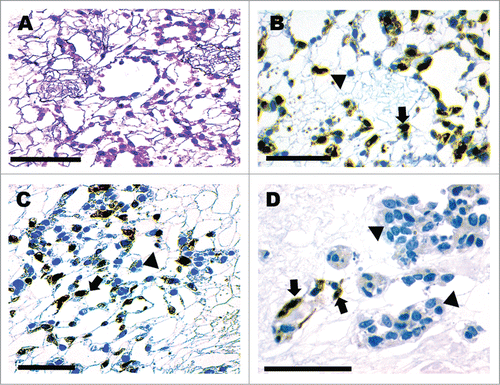
Figure 3. Three-day incubation of scaffold showing antegrade seeding of vasculature and retrograde seeding of collecting system. H&E (A). GFP+ cells line peritubular capillary vessels (B–D) while a GFP− βTC-tet cell population (D) occupy tubular structures. GFP+ cells are marked by arrows and the GFP− βTC-tet cells with arrowheads (B–D). Scale bar 100 μm.