Figures & data
Figure 1. Canonical Wnt signaling pathway. In the absence of signal, action of the destruction complex (CKIα, GSK-3β, APC, Axin) creates a hyperphosphorylated β-catenin, which is a target for ubiqitination and degradation by the proteosome. Binding of Wnt ligand to a Frizzled/LRP-5/6 receptor complex leads to stabilization of hypophosphorylated β-catenin, which interacts with TCF/LEF proteins in the nucleus to activate transcription. In a canonical pathway, CKIα, GSK-3β, APC, and Axin act as negative regulators and all other components act positively. Abbreviations: APC = adenomatous polyposis coli, CK = casein kinase, GSK = glycogen synthase kinase, Fzd = Frizzled-Rezeptor, LRP = low-density-lipoprotein receptor related protein, Tcf/Lef = T cell-specific transcription factor/lymphoid enhancer-binding factor.
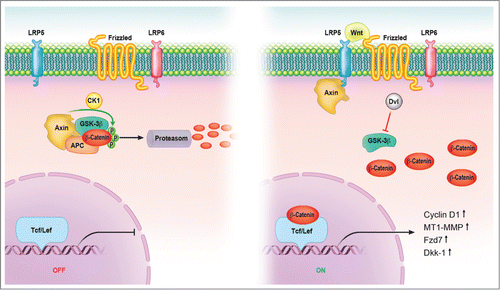
Figure 2. Wnt signaling maintains the hair-inducing activity in skin repair. Fibroblast growth factor (Fgf) 9 is a secreted signaling molecule that is expressed in epithelium. Mesenchymal Fgf signaling interacts with β-catenin-mediated Wnt signaling in a feed-forward loop that functions to sustain mesenchymal Fgf responsiveness and mesenchymal Wnt/β-catenin signaling. Wnt2a is a canonical Wnt ligand that activates mesenchymal Wnt/β-catenin signaling, whereas Fgf9 is the only known ligand that signals to mesenchymal Fgf receptors (FGFRs). Mesothelial Fgf9 and mesenchymal Wnt2a are principally responsible for maintaining mesenchymal Fgf-Wnt/β-catenin signaling, whereas epithelial Fgf9 primarily affects epithelial branching. In summary, Fgf signaling is primarily responsible for regulating mesenchymal proliferation, whereas β-catenin signaling is a required permissive factor for mesenchymal Fgf signaling. Abbreviations: Fgf = Fibroblast growth factor.
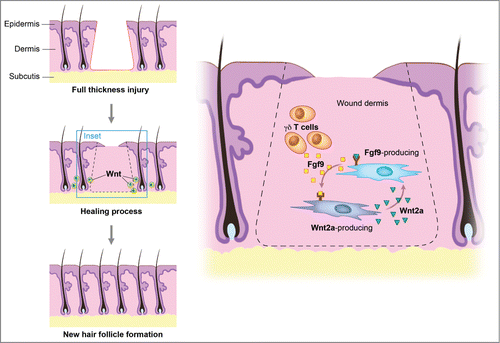
Figure 3. There are 3 classic stages of wound repair: inflammation (a), proliferation (b) and remodeling (c). (a) Inflammation. This stage lasts until about 48 h after injury and depicted is a skin wound at about 24–48 h after injury. The wound is characterized by a hypoxic (ischemic) environment in which a fibrin clot has formed and platelets aggregate. Platelets adhere to the injured endothelium and release chemokines, thereby attracting the cellular components of the inflammatory stage. The inflammatory stage of wound healing is characterized by the presence of neutrophils, macrophages, lymphocytes and local Wnt signaling begins to increase. The inflammatory cells then serve to release proinflammatory cytokines, growth factors and vascular endothelial growth factor, ingest foreign materials, increase vascular permeability, and promote fibroblast activity. (b) Proliferation. This stage occurs about 2–10 d after injury and depicted is a skin wound at about 5–10 d after injury. This stage includes an increased local Wnt response, capillary growth and granulation tissue formation occur and an eschar has formed on the surface of the wound. (c) Remodeling. This stage lasts for a year or longer and depicted is a skin wound about 1–12 months after repair. The final stage of wound healing is a long process of tissue remodeling and increasing wound strength. During this stage, type I collagen synthesis and turnover continues, and fibroblasts differentiate into myofibroblasts, allowing further wound contraction.
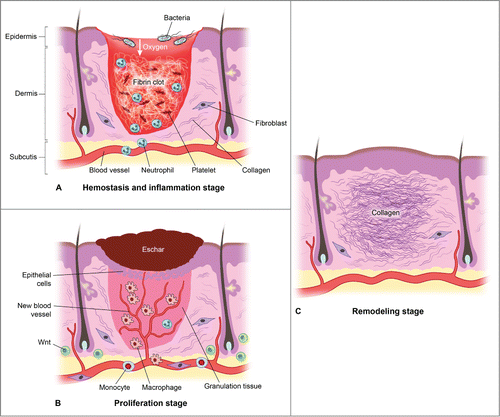
Table 1. Summary of developmental signaling pathways in mammalian skin development and repair
