Figures & data
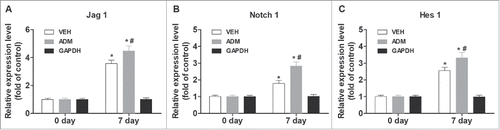
Figure 1. Notch/Jagged1 signaling pathway was enhanced during wound healing. To investigate the role of Notch/Jagged1 signaling pathway in wound healing and scar formation in vivo, we conducted our experiments using a mouse skin wound model to assess the expression of several key factors of Notch/Jagged1 signaling pathway using western blot. N = 10 mice *P < 0.05 compared with day 0.
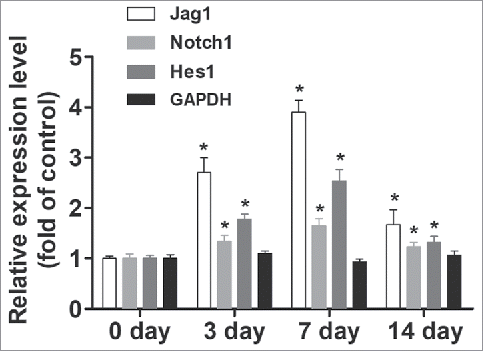
Figure 2. Jagged 1 inhibited the differentiation of ESCs to MFB in vitro. (A) Representative immunofluorescence staining. a) Hoechst counterstaining, b) Keratin 15, c) β1 Integrin, and d) merged image. (B) Western blot analysis of α-SMA expression in ESCs. (C) Western blot analysis of Collagen-I expression in ESCs. (D) Western blot analysis of Collagen-III expression in ESCs. *P < 0.05 compared with control.
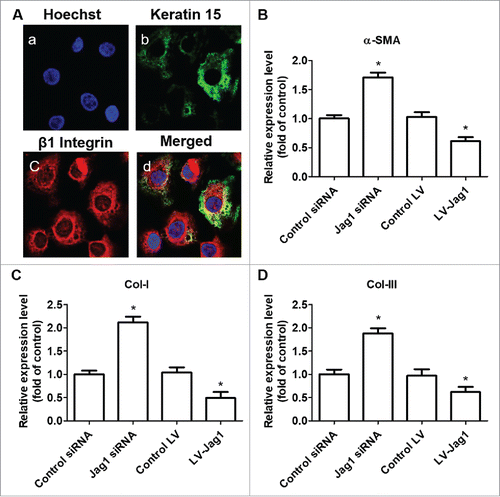
Figure 3. ESC-conditional knockout of Jag1 promoted scar formation. (A) Representative wound images, (B) Representative H&E staining, (C) Representative Masson staining. N = 10 mice/group.
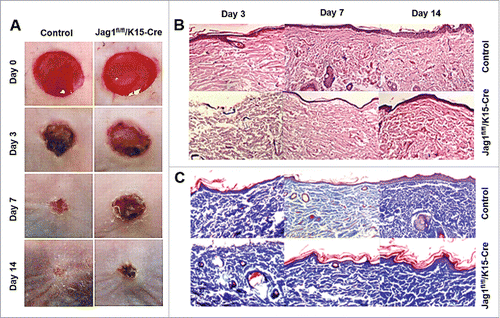
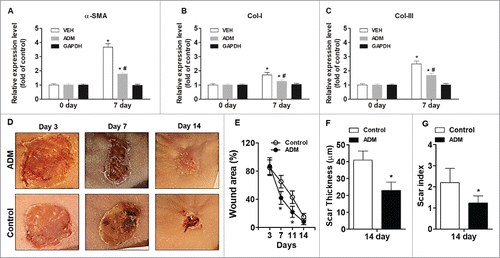
Figure 4. Porcine ADM treatment accelerated wound closure and reduced scar formation in vivo. (A) α-SMA expression in wound tissue at day 7. (B) Collagen-I expression in wound tissue at day 7. (C) Collagen-III expression in wound tissue at day 7. (D) Representative wound images. Images were taken after carefully removed porcine ADM. (E) Wound area (%) over time. (F) Scar thickness. (G) Scar index. N = 10 mice/group. (A, B, C) *P < 0.05 compared with day 0, #P < 0.05 compared with VEH. (E, F, G) *P < 0.05 compared with control.