Figures & data
FIGURE 1. Characteristics of hADSCs isolated by liposuction.
A, Morphological observation of hADSCs isolated by liposuction. The hADSCs cultured in 10% DMEM maintained a fusiform shape with oval nuclei that were arranged in a swirl pattern. Bar = 100 µm; B, Using the MTT assay, the proliferation of hADSCs cultured in DMEM with 10% FBS is shown. C, Flow cytometric analysis of CD markers expressed by hADSCs.
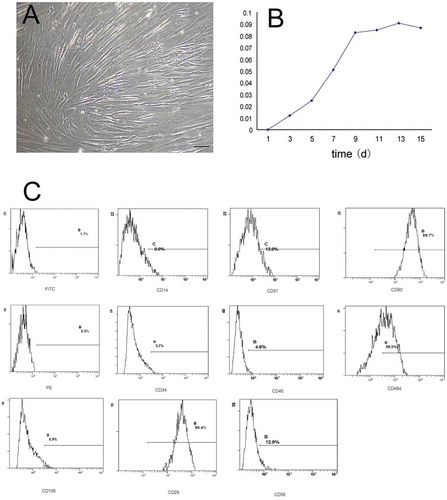
TABLE 1. Flow cytometric analysis of CD markers for hADSCs showed that cells that weakly expressed hematopoietic lineage markers (CD14, CD31, CD34, CD45, CD56, and CD106) and highly expressed lineage markers (CD49d and CD90) were not expressed in hematopoietic lineage cells but were in hADSCs
FIGURE 2. Biocompatibility evaluation of HPMC to hADSCs.
The morphology of HPMC hydrogels at different concentrations analyzed using a scanning electron microscope (SEM). The SEM assay was used to observe morphological characteristics of HPMC hydrogels at different concentrations. A, 10% (w/v) HPMC hydrogel exhibited tiny honeycomb-like features. B, The 13% (w/v) HPMC hydrogel showed clear cribriform patterns, and the void ratio was suited for cell proliferation and differentiation. C, The 15% (w/v) HPMC hydrogel showed lamellar structures. D, An SEM assay of hADSCs seeded with 13% (w/v) HPMC showed that the hADSCs were strongly attached to the HPMC with cytoplasmic extensions and lamellipodia. E, The cell viability of hADSCs seeded alone (blue group) or with an HPMC hydrogel at different concentrations (red group) was measured by CCK-8 assays; *p < .05 (independent samples group t-test) F, The cell proliferation of hADSCs seeded alone (blue group) or with 13% (w/v) HPMC hydrogel (red group) was evaluated at different time points using CCK-8 assays; *p < .05 (independent samples group t-test).
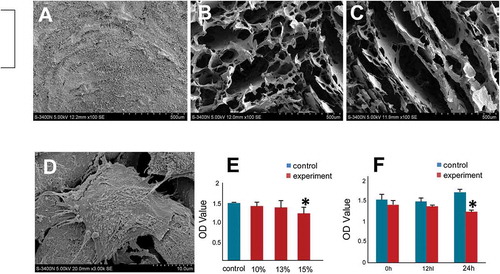
FIGURE 3. hADSCs differentiation into lymphocyte-like cells in vitro.
Immunocytochemistry results showed that passage 6 hADSCs cultured in the lymphocytic inducement medium for 14 days were strongly positive for lymphocytic markers including CD3, CD20, CD45RO, and CD79a compared with the control groups. Bar = 100 µm.
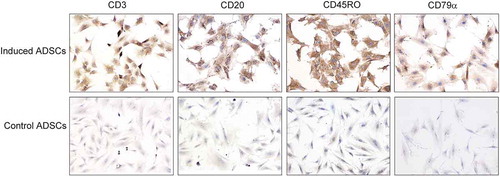
TABLE 2. The probability of cartilage-like tissues or lymph nodes formed in nude mice of different mixtures after injection for 8 weeks. Overall, 10/16 nodes were formed in the mixture 1 group, which contained hADSCs, HPMC, 15 µg/L TGF-β1 and 25 µg/L bFGF. In contrast, no nodes were observed in either the mixture 2 or 3 groups, which contained the same ingredients as mixture 1 but without cytokines (mixture 2) or HPMCs (mixture 3) (chi-square test, χ2= 6.348, p = .012)
FIGURE 4. hADSCs formed lymphoid nodules in vivo.
A, At 8 weeks after injection, a round nodule had formed subcutaneously under the injection site (black arrow) where passage 6 hADSC after induction were mixed with 13% (w/v) HPMC hydrogel. B, Hematoxylin and eosin staining showed a lymphoid node in the newly formed nodule, with clearly lymphoid follicle, deputy cortex, sinuses and other typical lymphoid microstructures inside the nodule. Bar = 100 µm. C. No nodule was observed in nude mice that were injected with passage 6 hADSC after induction, but without HPMC hydrogel. D. Hematoxylin and eosin staining showed that only degenerated and necrotic muscle tissues (black asterisk) were found at the injection sites where mixture 2 or 3 (without cytokines or HPMC) was injected. Bar = 100 µm.
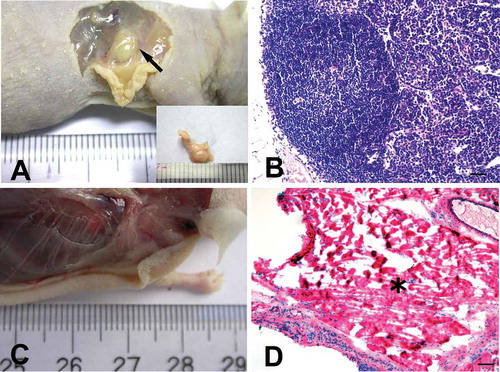
FIGURE 5. Immunohistochemical staining for T and B lymphocyte markers of the newly formed lymphoid nodules and spleens of the nude mouse compared with human markers.
In the newly formed node of the nude mice, lymphocyte-like cells were partly positive for the T cell markers CD3 and CD45RO between or inside the follicles, and some of the cells were weakly positive for B cell markers CD20 and CD79α that were mainly located inside the follicles. In human lymph node and spleen tissues, CD20- and CD79α-positive cells were mainly found inside the follicles, while CD3- and CD45RO-positive lymphocytes were mainly distributed throughout the interfollicular areas. No immunoreactivity was detected in spleens of the experimental nude mice. Bar = 50 µm.
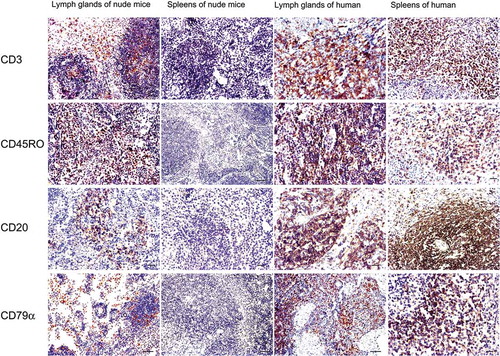
TABLE 3. The species identification report (No. 2007101) showed that the lymph nodes formed in the nude mice shared the same human DNA genetic markers as the human adipose-derived stromal cells (hADSCs) of the human donor
