Figures & data
Figure 1. In vitro culture of PEPCs showing a fast and robust growth after thawing. A cardiosphere-like structure (arrow) formed on top of spindle-like cells after 4 days culture. this photo was taken from one of the five patients’ PEPCs. all samples grew in a similar pattern. Bar = 10 µm.
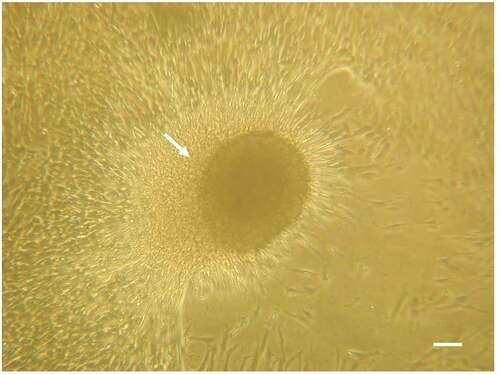
Figure 2. Decellularization removed cells from the pulmonary artery and retained the extracellular matrix. A) H-E-stained section of a non-decellularized porcine pulmonary artery revealing numerous cells in the intima, media, and adventitia. B). Immunofluorescent of nuclei DAPI stain in the same tissue. C) H&E stained section of the same vessel after decellularization. D) DAPI staining of the same section revealing total removal of cell nuclei. E, F) trichrome stain of the vessel before E) and after F) decellularization. The three layers of the vessel remained grossly intact after decellularization: an intact smooth intima, a compact media with red stained smooth muscle fiber, and collagen-rich adventitia and subintimal area. bar = 100 µm.
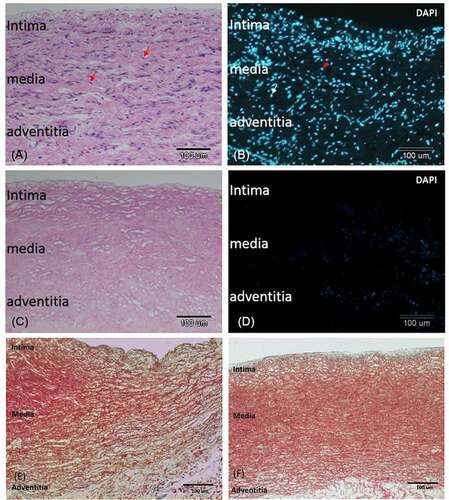
Figure 3. Images of decellularized scaffold in agarose gel. images A, C, and E are vertical projections. images B, D, and F are horizontal projections. In A) and B), the intima surface was facing upward (white arrow: intima; blue arrow: adventitia), whereas in C) and D), the adventitia surface was facing upward. In E and F, the pulmonary artery was cut into a 0.5 cm inner diameter × 0.5 cm long segment and a plastic microtip was insert in the vascular cannel to allow the cross section of the vessel facing upward at the center of a 12-well plate.
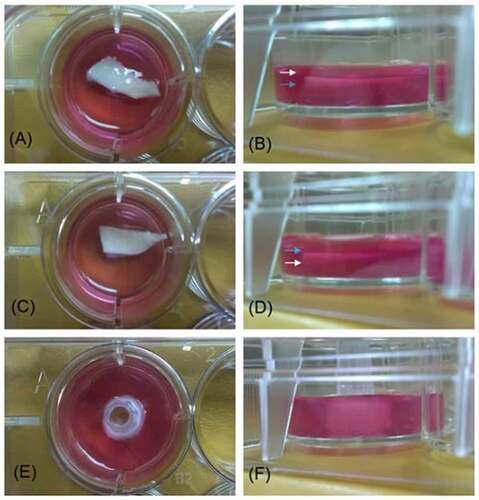
Figure 4. HS68 cells grew and migrated in the decellularized porcine pulmonary artery after implantation via different surfaces. Implantation via the A) adventitia, B) intima, C) lateral after 7 days, and implantation via the D) adventitia, E) intima, F) lateral after 14 days were shown with H&E and DAPI staining. arrows indicated the HS68 cells.
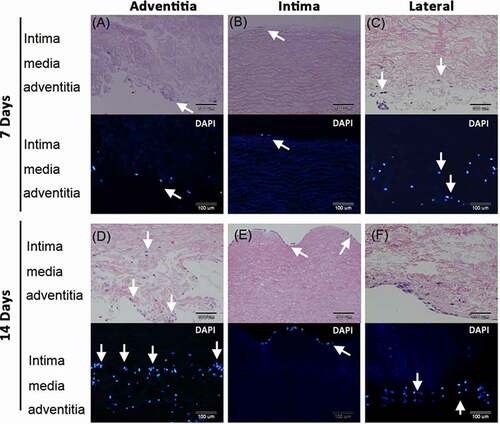
Figure 5. Human umbilical vein endothelial cells (HUVEC) grew and migrated in the decellularized porcine pulmonary artery after implantation via different surfaces. Implantation via the A) adventitia, B) intima, C) lateral after 7 days, and implantation via the D) adventitia, E) intima, F) lateral after 14 days were shown with H&E and DAPI staining. arrows indicated the HUVECs.
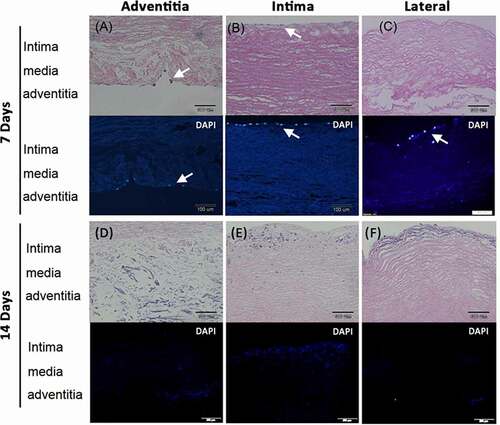
Figure 6. Pericardial effusion-derived progenitor cells (PEPCs) grew and migrated in the decellularized porcine pulmonary artery after implantation via different surfaces. Implantation via the A) adventitia, B) intima, C) lateral after 7 days, and implantation via the D) adventitia, E) intima, F) lateral after 14 days were shown with H&E and DAPI staining. arrows indicated the PEPCs.
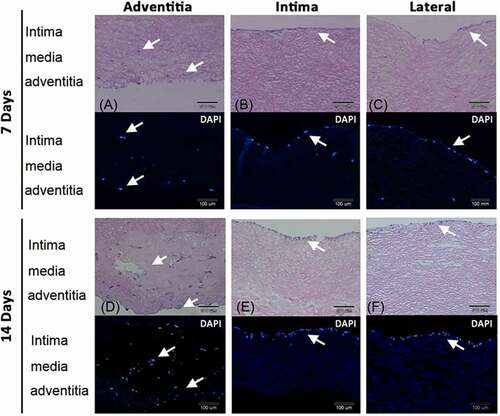
Figure 7. The cell number of HS68 fibroblasts, HUVECs, and PEPCs grew on a decellularized pulmonary artery pooling from 2 separate experiments. A) Day 7 after culture. B) Day 14 after culture (*p < .05, **p < .01, two-way ANOVA, bonferroni comparison test).
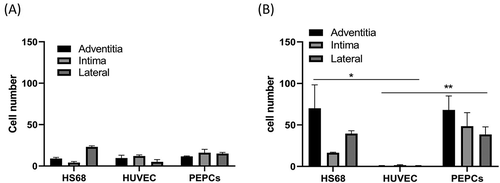
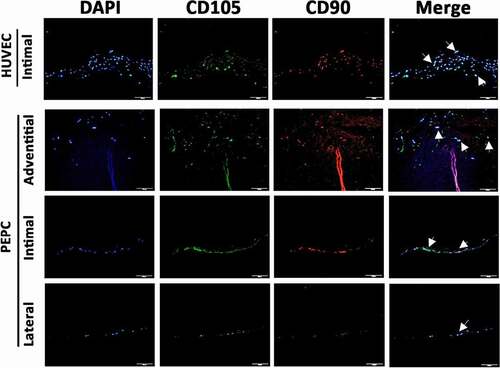
Figure 8. Pericardial effusion-derived progenitor cells (PEPCs) expressed vascular differentiation surface markers at day-14 after implantation on a decellularized porcine pulmonary artery. First row showed positive control of CD105 (green) and CD90 (red) expressions on HUVECs after implanting the cells on the intimal surface at day-14. PEPCs grew and migrated better when implanted on the adventitia surface (second row) as compared with implantation on intimal (the 3rd row) and lateral surfaces (the 4th row). All PEPCs expressed CD105 and CD90 after implantation. arrows indicated cells merged with all markers. Bar = 50 µm.