Figures & data
Figure 1. Core molecules in the Hippo pathway and their mode of action.
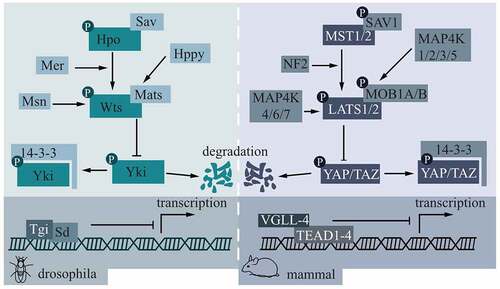
Figure 2. Hippo pathway controlling DPSCs.
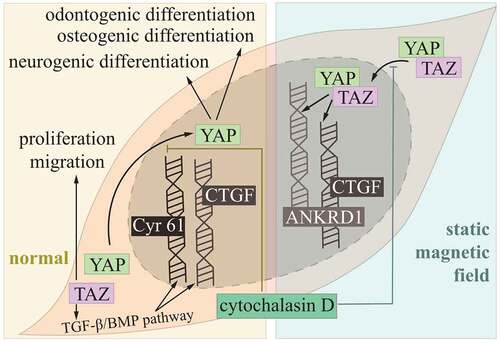
Figure 3. Hippo pathway controlling PDLSCs.
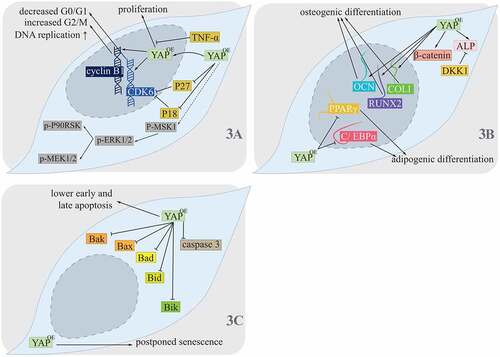
Figure 4. Development of enamel organ.
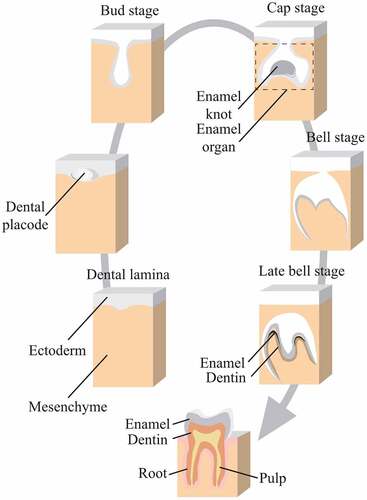
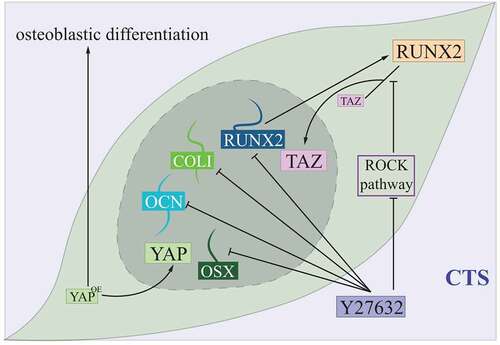
Figure 5. Hippo controlling PDLCs under the cyclic tensile stress condition.