Figures & data
Table 1. Published manufacturing protocols for regulatory T cells assessed in kidney transplantation phase I/II clinical trials.
Table 2. Published manufacturing protocols for regulatory T cells assessed in liver and heart transplantation phase I/II clinical trials.
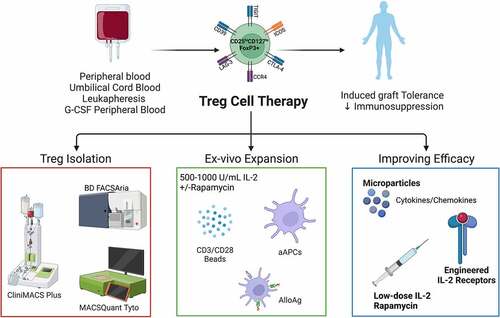
Figure 1. Overview of clinical manufacturing of regulatory T cell (Treg) products. Tregs can be isolated from peripheral blood, umbilical cord blood, leukapheresis, or G-CSF mobilized peripheral blood by magnetic cell separation and/or flow cytometric sorting using GMP grade closed systems. Isolated Tregs are then ex vivo expanded using anti-CD3/CD28 magnetic expander beads or artificial antigen-presenting cells (K562 64/86 aAPCs) in the presence of interleukin (IL)-2 with or without rapamycin. AlloAg-specific Tregs can be generated by culturing recipient Tregs with donor AlloAg-expressing APCs. Current in vivo and ex vivo strategies are being used to improve Treg ACT persistence and migration. Figure created with BioRender.com.