Figures & data
Figure 1. (A) Sm-class snRNAs are transcribed by RNA polymerase II as precursors that contain additional nucleotides at the 3′ end and a monomethylated m7GpppG (m7G) cap structure at the 5′ end. The pre-snRNA transcripts are then exported from the nucleus, often passing through the Cajal body. Once the pre-snRNAs translocate to the cytoplasm, the snRNA nuclear export complex dissociates. The SMN complex combines newly exported snRNAs with a set of Sm in a process known as Sm core assembly. Then, the snRNA is hypermethylated to form a 2,2,7-trimethylguanosine (TMG) cap structure in the pre-import complex. Finally, the RNP is imported back into the nucleus by specific snRNP import factors shown in B. Once in the nucleus, the partially assembled RNPs can transit through Cajal bodies where they undergo additional assembly and maturation steps. (B) The adaptor for the TMG cap is called snurportin1 (SPN1); this protein binds directly to the TMG cap and significantly improves the kinetics of snRNP import. SMN interacts with both SPN1 and Importin β. SMN functions to bridge the gap between the Sm core and the import machinery. These proteins, together with the snRNA and associated snRNP proteins, constitute the pre-import RNP complex.
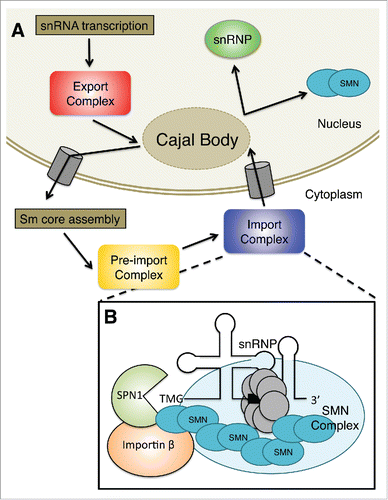
Figure 2. (A) In SMN(+) Cajal bodies, coilin and SMN physically interact. These heterotypic interactions are likely to regulate the overall composition of the nuclear body. Cajal bodies and Gems are kinetically autonomous compartments as illustrated by the Gem and SMN(−) Cajal body. The basis for this kinetic autonomy is likely due to the ability of SMN and coilin to maintain homotypic interactions (coilin-coilin and SMN-SMN) within the separated nuclear bodies. (B) SMN-Gem2 exists as a stable heterodimer that, for purposes of discussing higher order oligomerization, has been shown as a single structural unit (labeled “SMN”). At varying concentrations, this complex exists in an equilibrium mixture containing dimers, tetramers (dimer of dimers), and octamers of SMN-Gem2. Human SMN-Gem2 forms dimers to octamers and possibly even larger complexes. Octamers appear to form via self-association of tetramers. (C) The concentration of SMN within certain subcellular compartments (e.g. Cajal bodies, snRNA gene clusters or cytoplasmic stress granules) is significantly greater than that of the surrounding regions of the cell. High concentrations of SMN are also thought to be found in mRNP transport particles along axons as well as at neuromuscular junctions. If there are unique functions of SMN that can only be performed by higher-order oligomers it is likely that such functions are being carried out in specific cellular locales that contain high concentrations of the SMN complex, as illustrated by the bright green circles.
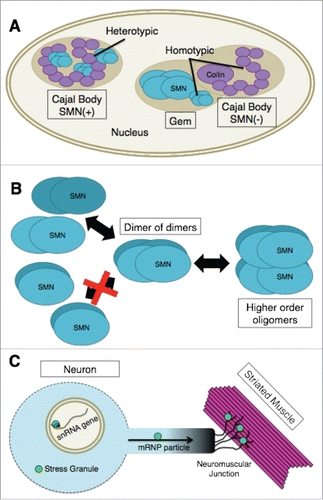
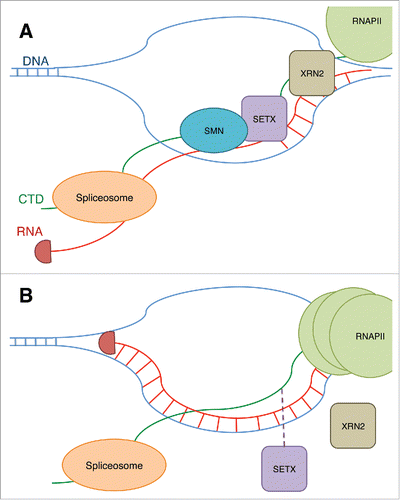
Figure 3. (A) The binding of SMN to R1810me2s on RNAPII CTD is shown to stabilize the binding of an RNA/DNA helicase, SETX, to the CTD. SETX prevents R-loops at termination sites and allows the exonuclease XRN2 to terminate the transcript, and RNAPII is released. The prevention of R-loops by SETX also allows the spliceosome to access the RNA and modify the transcript through splicing. (B) When SMN is reduced, or the R1810 residue is lost, this results in reduced recruitment of SETX and an increase in R-loops and DNA damage. RNAPII also accumulates at the transcription termination site.