Figures & data
Table 1. Stimulation protocols and associated LTF in the Aplysia bifurcated culture system.
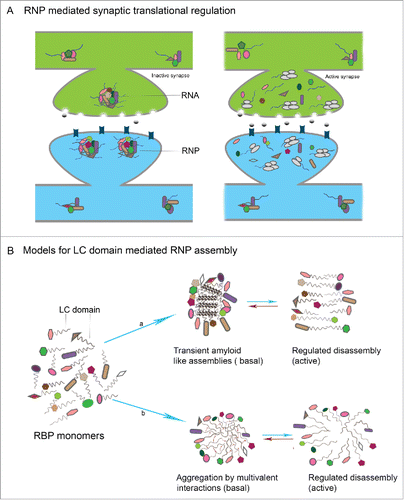
Figure 2. Prion mechanism of CPEB in memory (A) Mechanism of CPEB function in translational regulation: In the basal state CPEB interacts with Maskin or similar 4E-BPs which further interacts with eIF4E and prevents eIF4E interaction with eIF4G. Upon neuronal activity, CPEB gets phosphorylated and polyadenylation enables PABP association with eIF4G, which then disrupts 4E-BP-eIF4E interaction thereby permitting eIF4E-eIF4G interaction and resultant translation. (B) In the basal state both prionogenic and non-prion forms of CPEB exists mostly as monomers and can bind RNA and keep it in a translationally repressed state. Upon activity, the prionogenic form induces aggregation and the prion like nature induces self assembly and propagation of the aggregates where the RNA gets translated (C) CPEB monomers in the resting synapses and Q/N domain mediated amyloid like assemblies in active synapses.
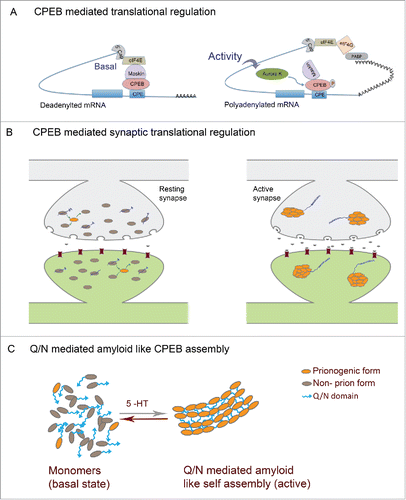
Figure 3. LC domain mediated RNP assembly in neuronal translational control (A) In the resting state, RNPs formed by interaction of individual RBPs and RNAs, sequester the RNA within RNPs in a translationally repressed state. Upon activity, the RNP disassembles leading to release of suppression resulting in translation of mRNAs with key synaptic functions (B) Models for LC mediated RNP assembly in neurons: in the resting state of synapses, individual RBPs interact by virtue of LC domains that are intrinsically disordered and form either a) transient and reversible amyloid like assemblies or b) reversible aggregates that are formed as result of weak multivalent interactions between LC domains.