Figures & data
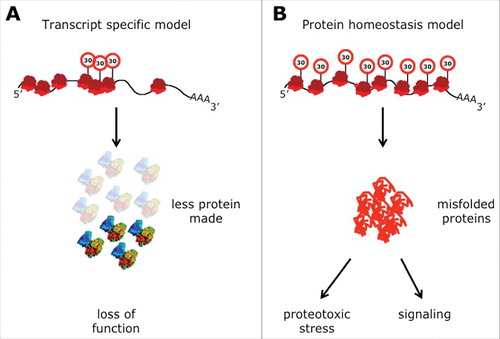
Figure 1. Modifications of wobble uridines (U34). (A) Uridine derivatives that can be found at the wobble position (U34) in anticodons of cytoplasmic tRNAs from eukaryotes. Modifications to uridine are indicated in red. (structures taken from ModomicsCitation21). Abbreviations: ncm5U: 5-carbamoylmethyluridine; ncm5Um: 5-carbamoylmethyl-2’-O-methyluridine; mcm5U: 5-methoxycarbonyl-methyluridine; mcm5s2U: 5-methoxycarbonylmethyl-2-thiouridine. (B) Detail of the structure of the anticodon stem loop (ASL) of human . mcm5s2U is highlighted by spheres. Sulfur (yellow), carbon (green), oxygen (red). Note that the nucleobases are arranged in a stacking confirmation (The structure is based on PDB 1FIR).
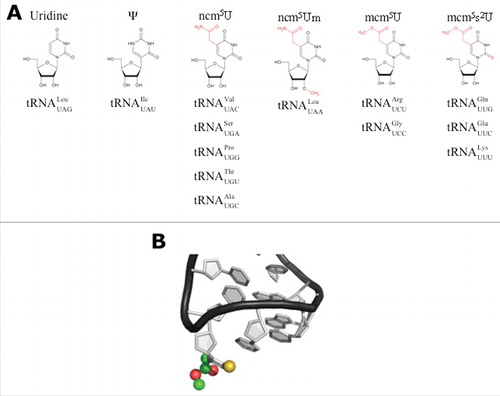
Figure 2. Model of the URM1 pathway and the Elongator complex. Schematic representation of the 2 pathways cooperating in mcm5s2U34 formation. In the URM1 pathway (left), sulfur is mobilized by Nfs1 with the help of Tum1. Uba4 activates Urm1, leading to a thiocarboxylate at Urm1's C-terminus, which acts as a sulfur carrier. Finally, the Ncs2•Ncs6 complex binds to and activates tRNA in the thiolation reaction and transfers the sulfur from Urm1 to uridine. The Elongator complex consists of twice Elp1-Elp6. Elp1 dimerizes via its C-terminus and acts as a platform for Elp2 and Elp3 binding in a wing-like structure. A ring of Elp4-Elp6 binds to one of the wings (Handedness is only partially represented in this model).Citation102,103
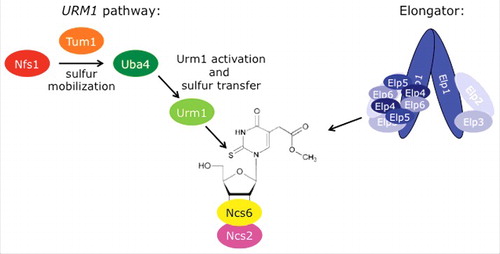
Figure 3. Phosphomodification of Elongator subunit Elp1. (A) Elp1 electrophoretic mobility shifts based on anti-HA Western blots are diagnostic for Elongator de-/phosphorylation.Citation130,134 In the kti12 and the kinase-dead hrr25/kti14 mutants, hypophosphorylated forms of Elp1-HA accumulate while sit4 phosphatase mutants induce Elp1-HA hyperphosphorylation. Wild-type (wt) cells maintain both isoforms of Elp1-HA, which mediate sensitivity (S) to growth inhibition by the tRNase toxin zymocin (killer assay: lower panel; for details see text). Zymocin resistance (R) associates with Elp1 phosphorylation defects in kti12, hrr25/kti14 and sit4 mutants. (B) Elongator phosphorylation model. Kti12 interacts with Elongator (and kinase Hrr25) thereby potentially activating Elp1 phosphorylation. In support of this, Elp1 is found to be hypophosphorylated in kti12 and hrr25/kti14 cells (see A). PPase: protein phosphatase (Sit4); Kinase: Hrr25/Kti14.
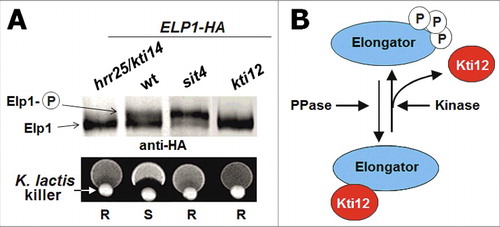
Figure 4. Two models to explain phenotypes of U34 modification mutants. (A) Specific mRNA enriched in codons that depend on tRNA modifications are translated at lower rates. This leads to reduced levels of the protein encoded by this transcript triggering a loss-of-function phenotype. (B) Ribosomes slow down when translating codons that depend on tRNA modifications. The slowdown perturbs the optimized equilibrium between speed of protein synthesis and protein folding. The increased rate of protein stress leads to a systemic failure in protein homeostasis and the aggregation of endogenous proteins that associates with it in a toxic gain-of-function scenario. This can either affect viability of the cells directly or by changing cellular signaling (Street signs with “30” indicate slow speed of ribosomes).