Figures & data
Figure 1. Multiple functions of CAMPs in host defense. CAMPs induce a variety of responses in host innate immune cells. They alter gene expression, induce production of chemokines and cytokines, promote recruitment of immune cells to the site of infection (chemotaxis), and inhibit growth of pathogenic species infecting the host. Selective control of inflammation in response to CAMP activity promotes wound healing and initiation of adaptive immune responses. LPS, lipopolysaccharide. Figure adapted from [Citation14,Citation15,Citation115].
![Figure 1. Multiple functions of CAMPs in host defense. CAMPs induce a variety of responses in host innate immune cells. They alter gene expression, induce production of chemokines and cytokines, promote recruitment of immune cells to the site of infection (chemotaxis), and inhibit growth of pathogenic species infecting the host. Selective control of inflammation in response to CAMP activity promotes wound healing and initiation of adaptive immune responses. LPS, lipopolysaccharide. Figure adapted from [Citation14,Citation15,Citation115].](/cms/asset/8c0d2175-2b35-4872-a895-63cca75141d5/krnb_a_1356980_f0001_b.gif)
Figure 2. Bacterial defenses against CAMPs and mechanisms for lowering the net negative charge of the cell envelope. CAMP resistance mechanisms include (1) efflux pumps, (2) CAMP-binding or hydrolyzing agents (3) formation of biofilms, and (4) addition of positively charged modifications to negatively charged substructures in the cell wall or lipid membrane. Addition of positively charged groups to the cell wall lowers the net negative charge of the envelope, decreasing binding of cationic compounds such as CAMPs and subsequently increasing bacterial resistance to these antimicrobials. In Gram-negative bacteria, lipid A in the outer membrane is the substrate for various positively charged modifications. Biosynthesis pathways for these modifications are not shown (For details see [Citation116-Citation118]). In certain species of both Gram-positive and Gram-negative bacteria, aminoacyl-phosphatidylglycerol synthases (aaPGSs) transfer aa from aa-tRNA (pre-formed by cytosolic aa-tRNA synthetases, aaRS) to phosphatidylglycerol (PG) in the membrane. In Gram positive bacteria, D-alanylation of lipoteichoic acid chains involves the enzymes encoded by the dltABCD operon. Positively and negatively charged molecules are represented in blue and red, respectively.
![Figure 2. Bacterial defenses against CAMPs and mechanisms for lowering the net negative charge of the cell envelope. CAMP resistance mechanisms include (1) efflux pumps, (2) CAMP-binding or hydrolyzing agents (3) formation of biofilms, and (4) addition of positively charged modifications to negatively charged substructures in the cell wall or lipid membrane. Addition of positively charged groups to the cell wall lowers the net negative charge of the envelope, decreasing binding of cationic compounds such as CAMPs and subsequently increasing bacterial resistance to these antimicrobials. In Gram-negative bacteria, lipid A in the outer membrane is the substrate for various positively charged modifications. Biosynthesis pathways for these modifications are not shown (For details see [Citation116-Citation118]). In certain species of both Gram-positive and Gram-negative bacteria, aminoacyl-phosphatidylglycerol synthases (aaPGSs) transfer aa from aa-tRNA (pre-formed by cytosolic aa-tRNA synthetases, aaRS) to phosphatidylglycerol (PG) in the membrane. In Gram positive bacteria, D-alanylation of lipoteichoic acid chains involves the enzymes encoded by the dltABCD operon. Positively and negatively charged molecules are represented in blue and red, respectively.](/cms/asset/c19680ac-a207-4683-9428-acd6dd5f14e5/krnb_a_1356980_f0002_oc.jpg)
Figure 3. Role of aa-tRNA transferases exhibiting a GNAT fold. aa-tRNA specificities are indicated in brackets. See text for details.
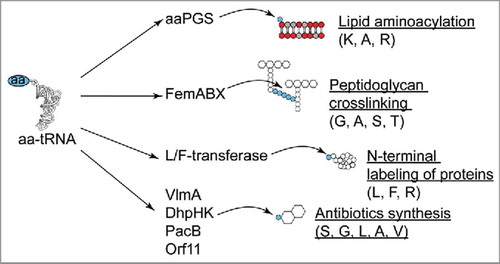
Figure 4. X-ray structures of the catalytic domains of AlaPGS from P. aeruginosa and LysPGS from B. licheniformis. A. Topology of the tandem GNAT fold repeat in aaPGSs. B. Structure of LysPGS with the Lys analog L-lysinamide bound to the aa binding pocket. Superposition of the FemX structure in complex with the tRNACitation50 reveal a possible binding mechanism of the tRNA, with helix 5 exhibiting basic residues. The cross-section of the structure shows the aa and PG binding pockets; the active site is located at the interface between the 2 pockets.
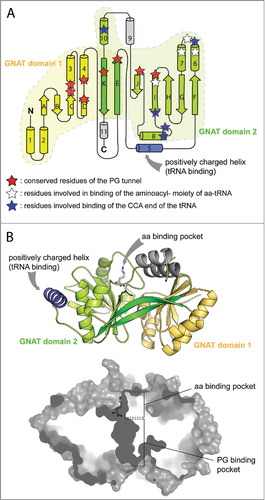
Figure 5. Lipid and aa substrates of lipid aminoacylation pathways. Phosphatidylglycerol (PG), Cardiolipin (CL), and diacylglycerol (DAG). See text for details.
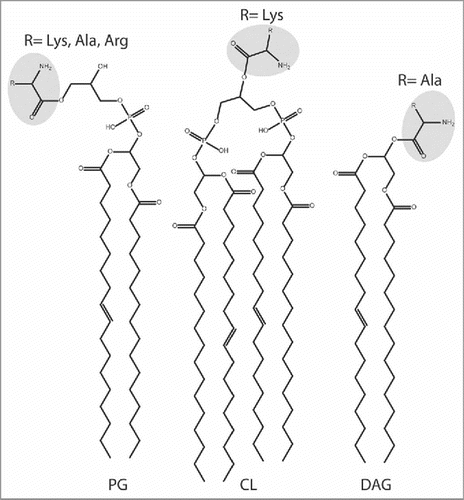
Figure 6. Homeostasis and utilization of aa-PG and Ala-DAG. A. In Corynebacterium, AlaDAGS utilizes Ala-tRNAAla and DAG to synthesize Ala-DAG. PesT, in concert with AlaDAGS, synthesizes Ala-PG. The role of PesT in this process is unknown.Citation55B. AhyD (in certain Firmicutes) and VirJ (in some proteobacteria) are aa-PG hydrolases. In B. subtilis, Lys-PG is N-succinylated by an unknown mechanism.
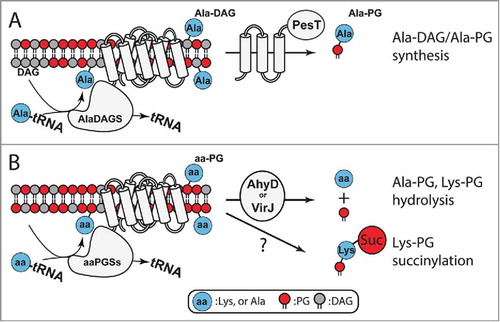
Figure 7. The 5-component signal transduction network controlling CAMP sensing and resistance pathways in S. aureus. CAMPs are sensed by VraFG and the signal is transduced to GraS through a mechanism that is likely to involve interaction between VraG and GraS. Activation of the GraSR system leads to increased transcription of the dltABCD operon and the mprF gene, leading to cell wall remodeling and CAMP resistance (figure adapted from Citation[107]).
![Figure 7. The 5-component signal transduction network controlling CAMP sensing and resistance pathways in S. aureus. CAMPs are sensed by VraFG and the signal is transduced to GraS through a mechanism that is likely to involve interaction between VraG and GraS. Activation of the GraSR system leads to increased transcription of the dltABCD operon and the mprF gene, leading to cell wall remodeling and CAMP resistance (figure adapted from Citation[107]).](/cms/asset/ab636924-e1e2-4909-bc75-b931a4cf83c6/krnb_a_1356980_f0007_oc.jpg)
