Figures & data
Table 1. DIS3L2-mediated RNA decay and corresponding biological function it impacts.
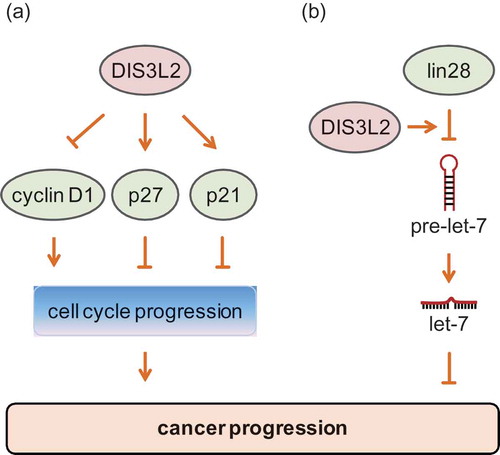
Figure 1. Structure of human DIS3L2. Human DIS3L2 contains two CSD domains (CSD1, CSD2), one RNB domain, and one S1 domain. Both the CSD domain and the S1 domain are RNA binding domains. The RNB domain provides 3ʹ-5ʹ exoribonuclease activity and the aspartic acid at position 391 (D391) is a catalytic site, whose mutation leads to inhibition of DIS3L2’s exoribonuclease activity.
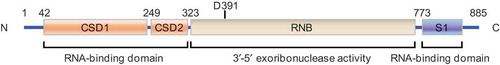
Figure 2. Function of DIS3L2 during cancer progression. (a) DIS3L2 suppresses the expression of cyclin D1 but enhances the expression of p27 and p21, therefore promoting the cell cycle progression and functioning as an inhibitor during cancer progression. (b) DIS3L2 promotes lin28-mediated suppression of pre-let-7, results in reduced let-7 biogenesis, and facilitates cancer progression.