Figures & data
Figure 1. Overview of covalent and transient labelling techniques for RNA and RNP labelling. For each approach, the methods chosen can be combined to obtain RNAs or RNPs with multiple site-specific modifications.
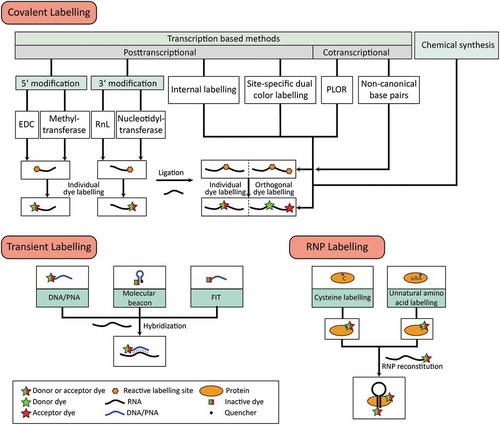
Figure 2. Available modifiable building blocks for solid-phase synthesis. Attachment sites for all four natural nucleotides are shown, as well as attachment sites on the ribose moiety.
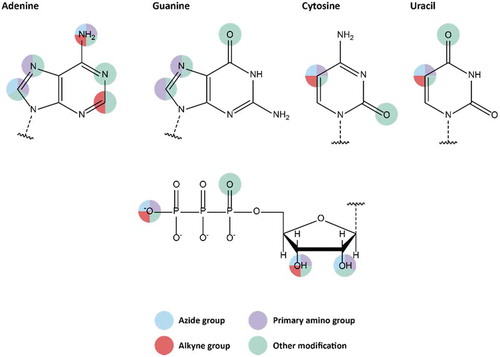
Figure 3. Chemoenzymatic labelling techniques for site-specifically attaching fluorophore labels to RNAs. a: Splinted ligation of commercially synthesized, fluorophore-labelled RNAs. b: DNAzyme-mediated addition of modified GTP. c: 5ʹ-end-labeling using carbodiimide-based chemistry. d: 5ʹ-guanosine-labeling using the mRNA modifying methyltransferase with modified S-adenosylmethionine analogues. e: Labelling using a terminal deoxynucleotidyl transferase, and subsequent ligation. f: Internal labelling by 3ʹ-modification using a modified pNp substrate, and subsequent ligation. g: Dual-colour labelling using targeting strand for internal labelling, together with periodate-based functionalization of the 3ʹ-end.
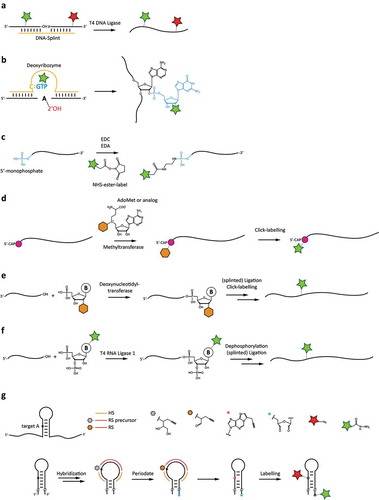
Figure 4. Cotranscriptional functionalization or modification approaches. a: Modification based on genetic code expansion using non-natural base pairs. b: Reaction scheme of the PLOR technique employing reinitiated transcription reactions. c: Integration of RNA sequences binding profluorophores for labelling.
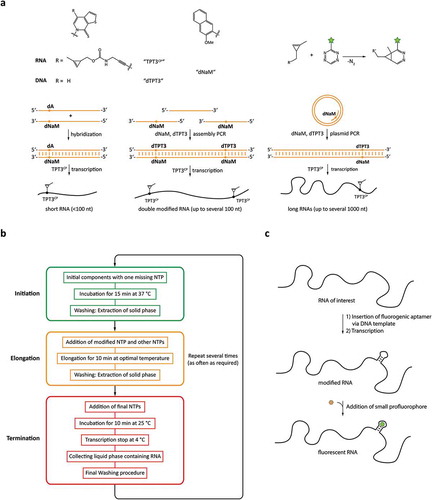
Table 1. Commercially available fluorescent dye for different modification strategies and chemical coupling approaches.
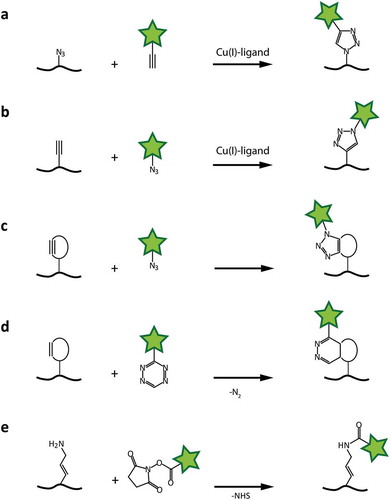
Figure 5. Chemical coupling reactions for fluorophore attachment to RNAs. a and b: Cu-catalyzed 3 + 2-cycloaddition, with azide moiety either in the RNA or on the fluorophore. c: 3 + 2-cycloaddition with strained alkyne. d: Cycloaddition using a strained diene (i.e. norbornene) and tetrazine. e: N-hydroxy-succinimide coupling to primary amines.