Figures & data
Figure 1. Expression and ribosome association of the tRNAPro half in different mammalian cell lines. (A) Northern blot analysis of the tRNAPro 5ʹ half of 20 µg of total RNA or 20 µg of RNA isolated from the crude ribosome pellet (P100) from CHO-K1 at several stress conditions: heat shock (heat), oxidative stress (oxid), oxidative stress with recovery, stationary (stat) growth phase and as a control exponential (exp) growth phase. Lower panel shows the same northern blot in the region of the tRNA half with increased contrast. (B) Northern blot analysis of the tRNAPro 5ʹ half of 30 µg total RNA isolated from HeLa, HEK, BON, NCI, and Hep3B cells. Ethidium bromide-stained 5 S rRNA serves as a loading control. (C-E) Polysome profiling of exponentially grown CHO-K1, HeLa, and HEK cells, respectively (top panels). RNA was isolated from the indicated fractions (free RNA, 40 S, 60 S, 80 S, and polysomes), its integrity, and identity monitored by agarose gel electrophoresis (middle panel) and used for northern blot analysis (lower panels). Full length (FL) tRNAPro and the tRNAPro 5ʹ half are indicated.
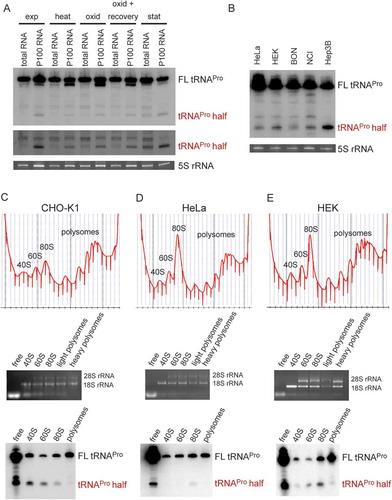
Figure 2. Binding of the tRNAPro half to ribosomes and ribosomal subunits in vitro. (A) Binding of the P32-labelled tRNAPro 5ʹ half to the crude ribosomal pellet (P100) and gradient-purified ribosomal subunits (40 S and 60 S) isolated from CHO cells, HeLa, and yeast cells was monitored by filter binding. Negative control (-) shows background signals in the absence of ribosomal particles. An unrelated small ncRNA (control RNA) from H. volcanii showed no (HeLa, yeast) or severely reduced (CHO) binding to ribosomal particles and serves as a binding specificity control. (B-C) Photocrosslinking of the tRNAPro half to human ribosomes. 5ʹ P32 – labelled tRNAPro half-carrying 4-thio-uracils was crosslinked to crude HeLa ribosomes at 366 nm followed by protease K treatment and RNA isolation. (B) After photo-crosslinking, RNAs were resolved on a 1% agarose gel. The ethidium bromide-stained gel and the autoradiogram thereof are shown. 18S rRNA and 28 S rRNA are labelled. (C) After photo-crosslinking, purified RNAs were subjected to RNase H treatment guided by DNA oligos complementary to different regions of 18S rRNA and the cleavage products were separated on 4% polyacrylamide denaturing gels. Ethidium bromide staining and autoradiogram are shown. Expected sizes of the produced rRNA fragments are indicated above each lane.
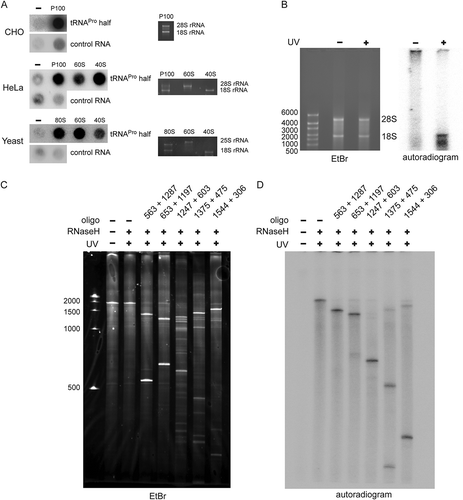
Figure 3. Effect of tRNAPro 5ʹ half on translation. (A) Autoradiogram of an SDS polyacrylamide gel after in vitro translation in the presence of the synthetic tRNAPro half using a CHO cell lysate. (B) Quantification of six in vitro translation experiments performed with CHO cell lysates in the absence (-) or presence (+) of the tRNAPro half. Values were normalized to the in vitro translation reaction with no tRNAPro half added. Box shows ± 25% of the mean, error bars show standard deviation. (C) Autoradiogram of an SDS polyacrylamide gel after in vitro translation of one reporter in the absence (-) presence of various synthetic tRNA 5ʹ halves (5 or 8 µM) using a rabbit reticulocyte cell lysate. In all cases, the tRNA 5ʹ halves are abbreviated by their respective amino acid three letter code. The asterisk marks the position of the full-length reporter protein. (D) Quantification of three in vitro translation experiments performed with rabbit reticulocyte cell lysate and one mRNA reporter in the presence of different tRNA 5ʹ halves (5 or 8 µM). Values were normalized to the in vitro translation reaction in the absence of the tRNAPro half. The horizontal lines indicate the mean and the error bars show standard deviations. (E) CHO in vitro translation in the presence of 8 µM synthetic tRNA halves. The apparent tRNAPro half-induced product (ProTiP) is indicated with a black arrowhead (here and in all panels). (F) CHO in vitro translation with increasing amounts of tRNAPro half added. (G) Quantification of ProTiP formation of three in vitro translation reactions as a function of increasing tRNAPro 5ʹ half concentration. The horizontal lines indicate the mean and the error bars show standard deviations. In (A), (C), (E), and (F) the coomassie stained protein bands of the lysate serve as a loading control. In (B) and (D) significant differences were determined using the 2-tailed paired Student’s t-test (***p < 0.001, **p < 0.01, *p < 0.05).
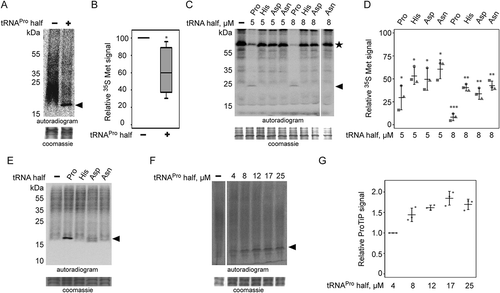
Figure 4. Effect of the tRNAPro half on translation in different eukaryotic systems. Autoradiograms of in vitro translation reactions performed using (A) HeLa, (B) HEK and (C) yeast extracts in the absence or presence of the tRNAPro 5ʹ half. Cycloheximide (CHX) with an f.c. of 7.5 mg/ml was used to inhibit global translation. ProTiP is indicated with a black arrowhead. (D) In vitro translation using CHO extracts and ProTiP formation (arrow head) was monitored in the presence of increasing amounts of cycloheximide (CHX). (E) Formation of ProTiP in vivo. 300 pmol of synthetic tRNAPro half was transfected into HeLa cells and the cells were subsequently incubated with S35 methionine. RNA isolated after metabolic labelling performed with (+) or without (-) transfected tRNAPro half was separated on a denaturing gel and visualized via autoradiography. The ethidium brome (EtBr) strained gel serves as a loading control. (F) Autoradiogram of in vitro translation reactions with HeLa ribosome-containing pellets (P100) or the corresponding ribosome-free supernatants (S100). Newly synthesized proteins, as well as ProTiP (black arrowhead), were produced only once P100 and S100 were combined. 100 pmol of tRNAPro half was used. (G) Inhibition of global translation in CHO extracts and ProTiP formation in the absence (-) or presence (+) of harringtonine (f.c. 2.5 mg/ml). In (A), (B), (C), (D), (F), and (G) the coomassie stained protein gels serve as loading controls.
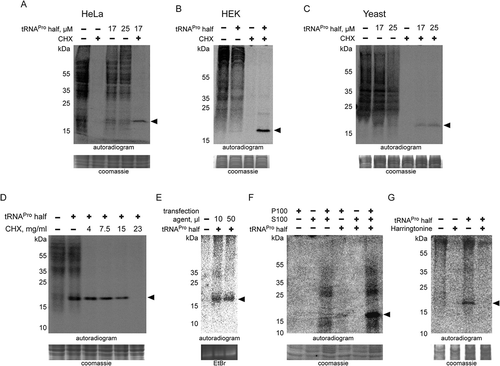
Figure 5. Chemical nature of ProTiP. (A) The products of in vitro translation using CHO cell lysates were incubated with proteinase K or RNase I. Protease K digests all newly translated and cell lysate proteins, as well as ProTiP (arrowhead). Also, RNAse I digestion removes ProTiP. The coomassie or ethidium bromide-stained gels serve as controls for efficient protein and RNA digestions, respectively. (B) Spike-in of yeast bulk tRNA (lane 3) after CHO in vitro translation in the absence (lane 1) or presence (lane 2) of 8 µM tRNAPro 5ʹ halves shows that ProTiP (arrowhead) migrates in the size range of tRNAs in the SDS polyacrylamide gel used. The gel was stained with coomassie (loading control) or with ethidium bromide (for visualization of the spiked-in tRNAs). (C) In vitro translation reactions (with or without tRNAPro halves) were loaded before (-) or after (+) phenol/chloroform/isoamyl alcohol (PCI) extraction on a denaturing SDS gel. ProTiP (black arrowhead) can be extracted into the water phase during PCI treatment. (D) Autoradiogram of an SDS polyacrylamide gel after in vitro translation in the presence of the internally 32P-labelled tRNAPro half (open arrowhead). Migration of the labelled tRNAPro half did not change after reactions in the presence of the cell lysate and the translational mix, thus demonstrating that ProTiP does not contain the tRNAPro 5ʹ half. Expected size of ProTiP is indicated with the black arrowhead.
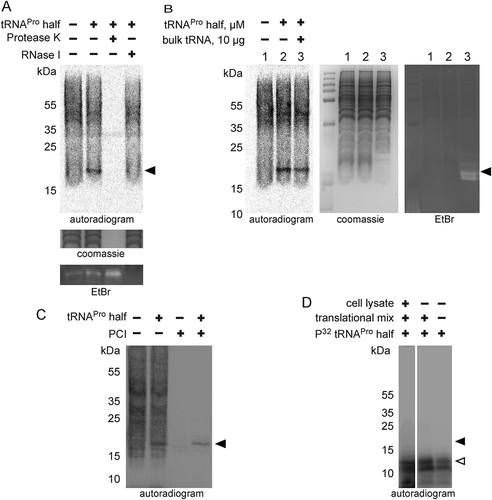
Figure 6. ProTiP is likely a peptidyl-tRNA. (A) Autoradiogram and ethidium bromide staining of an acidic gel after in vitro translation performed in the absence (-) or presence (+) of the tRNAPro 5ʹ half. Charged tRNAiMet and tRNAMet are labelled by asterisks. ProTiP is indicated with the black arrowhead. (B) Autoradiogram of an 8% denaturing polyacrylamide gel showing the position of ProTiP (arrowhead) before (-) and after (+) polyadenylation. Middle and left panels: Ethidium bromide staining and northern blot against 5.8S rRNA demonstrates the efficiency of polyadenylation. (C) Copper sulphate treatment of RNA isolated after in vitro translation performed in the presence (+) or absence (-) the tRNAPro half. The location of ProTiP, which was insensitive to copper sulphate treatment, is indicated in the autoradiogram with the black arrowhead. The Met-tRNAMet molecules are labelled with asterisks. (D) Fractions of the polysome profile after in vitro translation in the presence of the tRNAPro half were subjected to isopropanol precipitation. Precipitated RNA was separated on a denaturing gel and visualized via phosphorimaging. ProTiP is indicated with the black arrowhead. (E) Binding of ProTiP to purified HeLa 80 S in vitro with and without pre-incubated bulk tRNAs. Ribosome-bound 35S-ProTiP was determined by filter binding. (F) Quantification of three individual binding experiments. Values were normalized to the binding of ProTiP to ribosomes without the addition of bulk tRNAs. The error bars show standard deviations (*p < 0.05).
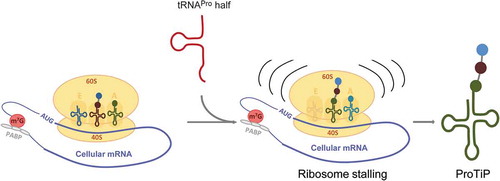
Figure 7. Model of the effect of the tRNAPro 5ʹ half on translation. The tRNAPro is processed into the tRNAPro 5ʹ half through cleavage at the anticodon region. The tRNAPro 5ʹ half binds to the ribosome and likely causes ribosome stalling. Consequently, translation is inhibited globally resulting in the accumulation of the peptidyl-tRNA inside the stalled ribosomes that we identified as ProTiP.