Figures & data
Figure 1. Obvious carrier effects on intercellular miRNA transfers have been vastly overlooked and thus are ‘The Elephant in the Room’. The carrier-delivering and carrier-acting aspects of exosomal transfer of miRNAs to targeted cells are predicted to promote the transfer and subsequent molecular functions of their delivered miRNA on the targeted cells. This can account for unexpected greater effects of exosome miRNA delivery and subsequent intracellular actions, than conceptualized by established canonical concepts of miRNA of only direct addition to targeted cells or genetic expression of miRNAs. Thus, exosome transfers of what are considered low amounts of miRNA, acting via both ‘carrier-delivery’ and ‘carrier-acting’ modes are the ‘Elephant in the Room’. This is because these obvious exosome carrier effects have been overlooked by both the miRNA Cognoscente clinging to conventional miRNA transfer concepts and thus, not considered as crucial to miRNA transfers to acceptor cells, and by the few remaining anti-exosome Luddites, who doubt the significance of EV per se. These carrier ‘Elephant in the Room’ aspects of these issues should be obvious, but are overlooked because it makes some uncomfortable, who are clinging to conventional canonical concepts; as out of this box (non- canonical). The point is that something as conspicuous as an elephant can be overlooked amid codified canonical concepts of such interactions

Figure 2. A. Oral and other systemic routes of administration of CD8pos suppressor T cell-derived exosomes strongly inhibit cutaneous OVA protein-induced DTH in actively immunized hosts. The Figure shows that oral (PO) administration of a single physiological dose of suppressive exosomes (1010 vesicles) from CD8+ suppressor T cells from ovalbumin (OVA) protein antigen (Ag) immune high Ag dose tolerized mice are inhibitory of active T cell mediated delayed-type hypersensitivity (DTH) elicited by on day 4 after optimal immunization by intradermal OVA Ag injection of only five micrograms into the ears of murine hosts immunized with an optimal 100 µg dose of OVA intradermally. Further, this single systemic transferred physiological dose of Ag tolerance induced inhibitory exosomes, can last very significantly for at least 120 h (4 days), when such systemic treatment is begun just after the peak of the maximal elicited 24 h active ear swelling response of the recipients. The Figure shows that the strong and prolonged suppressive results of administered suppressive exosomes are similar among various conventional systemic treatment routes (IP, intraperitoneal, green line; IV, intravenous, blue line; and even ID, intradermal, black dashed line), that all very strongly inhibit elicited existing active DTH by 50–90% over the subsequent 4 days (120 h); with oral administration the most profoundly suppressive (red line). B. CASEIN milk protein-induced active in vivo elicited DTH, is suppressed by Casein-specific B1a B cell, miRNA-150-associated suppressor exosomes, given systemically by various routes, in a single physiological dose at the 24 h peak of response; lasts at least for 4 days; especially the most powerful inhibitory response to oral exosome administration. Suppression of delayed-type hypersensitivity (DTH) to soluble casein (Cas) Ag by miRNA-150- associated B1 B cell-derived small exosome EVs of Cas-Ag-immunized mice. For supernatant exosomes, mice had been intradermally (ID) immunized with saline solution of soluble Cas antigen 3 days before harvest of lymph nodes and spleens containing Cas-immune B1 B cells, that were cultured for a subsequent 48 h. The resulting supernatant was ultra-filtered and then ultracentrifuged to concentrate Cas-specific B1a B cell-derived exosome extracellular vesicles, that then were supplemented with miRNA-150 by association in vitro at 37° C with miRNA-150 and then washed free of unattached miRNA-150 by another round of 100,000 g ultracentrifugation. Mice that had been optimally ID immunized with saline solution of soluble Cas antigen (100 μg per mouse) 5 days before challenging by ID ear administration of the same Cas solution (at only 5 μg per ear lobe). Then, after measurement of the 24-h ear swelling responses, miRNA-150-supplemented, Cas-Ag specific B1a B cell-derived exosome EVs were administered either intraperitoneally (IP, purple line), ID (green line), intravenously (IV, orange line) or orally (PO, redline), and subsequent ear swelling responses were measured daily up to 120 h post ear challenge, and expressed as delta ± SEM [units (U) × 10−2 mm]; all compared to the control treated group (black line). All routes of administration were suppressive, but the oral route clearly was the most powerful
![Figure 2. A. Oral and other systemic routes of administration of CD8pos suppressor T cell-derived exosomes strongly inhibit cutaneous OVA protein-induced DTH in actively immunized hosts. The Figure shows that oral (PO) administration of a single physiological dose of suppressive exosomes (1010 vesicles) from CD8+ suppressor T cells from ovalbumin (OVA) protein antigen (Ag) immune high Ag dose tolerized mice are inhibitory of active T cell mediated delayed-type hypersensitivity (DTH) elicited by on day 4 after optimal immunization by intradermal OVA Ag injection of only five micrograms into the ears of murine hosts immunized with an optimal 100 µg dose of OVA intradermally. Further, this single systemic transferred physiological dose of Ag tolerance induced inhibitory exosomes, can last very significantly for at least 120 h (4 days), when such systemic treatment is begun just after the peak of the maximal elicited 24 h active ear swelling response of the recipients. The Figure shows that the strong and prolonged suppressive results of administered suppressive exosomes are similar among various conventional systemic treatment routes (IP, intraperitoneal, green line; IV, intravenous, blue line; and even ID, intradermal, black dashed line), that all very strongly inhibit elicited existing active DTH by 50–90% over the subsequent 4 days (120 h); with oral administration the most profoundly suppressive (red line). B. CASEIN milk protein-induced active in vivo elicited DTH, is suppressed by Casein-specific B1a B cell, miRNA-150-associated suppressor exosomes, given systemically by various routes, in a single physiological dose at the 24 h peak of response; lasts at least for 4 days; especially the most powerful inhibitory response to oral exosome administration. Suppression of delayed-type hypersensitivity (DTH) to soluble casein (Cas) Ag by miRNA-150- associated B1 B cell-derived small exosome EVs of Cas-Ag-immunized mice. For supernatant exosomes, mice had been intradermally (ID) immunized with saline solution of soluble Cas antigen 3 days before harvest of lymph nodes and spleens containing Cas-immune B1 B cells, that were cultured for a subsequent 48 h. The resulting supernatant was ultra-filtered and then ultracentrifuged to concentrate Cas-specific B1a B cell-derived exosome extracellular vesicles, that then were supplemented with miRNA-150 by association in vitro at 37° C with miRNA-150 and then washed free of unattached miRNA-150 by another round of 100,000 g ultracentrifugation. Mice that had been optimally ID immunized with saline solution of soluble Cas antigen (100 μg per mouse) 5 days before challenging by ID ear administration of the same Cas solution (at only 5 μg per ear lobe). Then, after measurement of the 24-h ear swelling responses, miRNA-150-supplemented, Cas-Ag specific B1a B cell-derived exosome EVs were administered either intraperitoneally (IP, purple line), ID (green line), intravenously (IV, orange line) or orally (PO, redline), and subsequent ear swelling responses were measured daily up to 120 h post ear challenge, and expressed as delta ± SEM [units (U) × 10−2 mm]; all compared to the control treated group (black line). All routes of administration were suppressive, but the oral route clearly was the most powerful](/cms/asset/0ccc3c98-7c8b-4729-ac0f-95e05bb3a0c1/krnb_a_1885189_f0002_c.jpg)
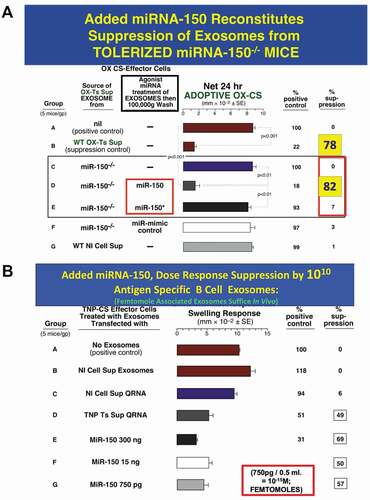
Figure 3. A. Added miRNA-150 reconstitutes suppression mediated by exosomes from Ag-tolerized mRNA-150−/- mice. miRNA-150−/- mice show definitively that miR-150 is the suppressive entity in the exosome-like nanovesicles derived from CD8+ suppressor T cells of high dose Ag-tolerized mice. Nanovesicle small exosomes from OX-hapten-Ag tolerized miRNA-150−/- mice associated by in vitro incubation at 37° C with added miR-150, mediated suppression of immune ear swelling responses (Group D, 82% suppression) like exosomes from wild type Ag-tolerized mice (Group B, 78% suppression), both vs. Group A positive wild type controls, 0% suppression), whereas exosomes associated instead with control miRNA-150* (Group E) or with miRNA-mimic control (Group F) did not result in suppression; respectively 3% and 1% suppression.
B. Added miRNA-150 for dose response suppression by 1010 Ag-Specific B1a B cell-derived exosomes; femtomole associated exosomes suffice in vivo. Suppression of CS immune ear swelling responses by extracellular miRNA from TNP Ag-specific Ts cell-derived supernatant that was freed from lysed exosomes, or a dose response of miR-150 added alone, that were associated with B1a B-cell derived Ag-specific exosomes. Treatment of the TNP Ag- specific-CS-effector mixed cells (shown alone in Group A) with 2 day immune B1a B cell-derived TNP-Ag-specific exosomes that were associated by in vitro incubation at 37° C with the soluble Qiagen RNA fraction from TNP CD8 + T suppressor cell-derived exosomes (Group D) vs. control normal cell derived exosomes (Group B), or similar extract from normal cell exosomes (Group C), results in significant suppression of the adoptive immune cell transfer of an OX Ag-specific CS effector cell mixture (49% suppression vs 0–6% suppression of the control groups A, B and C). Similarly, 2 day immune Ag-specific B-1a B cell-derived exosomes, supplemented with serial decreasing doses of miR-150 alone, mediated suppression of TNP-CS-effector cell adoptive transfer of CS ear swelling responses (Groups E, F and G), down to a dose of 750pg miRNA-150 added per eventual recipient, which is 50 femtomoles per eventual recipient (Group G). Similar results were obtained in two other experiments in the CS system