Figures & data
Figure 1. Common chemical modifications used in RNA-based therapeutics. Nuclease resistance of ASOs is improved by substituting the parent phosphodiester bonds with phosphorothioate (PS) linkages in which a sulphur atom replaces one of the non-bridging oxygen atoms in the phosphate group. In addition, PS modifications enhance plasma protein binding and reduce clearance of the ASOs, thereby enhancing the PK properties of PS-ASOs. Improved stability and increased binding affinity is achieved using nucleotides with sugar modifications, including different 2’-modified sugars such as the 2’-O-methyl (2’-O-Me), 2’-O-methoxyethyl (2’-MOE), or 2ʹ-fluoro (2’-F) modifications, or the bicyclic locked nucleic acid (LNA) modification, in which the ribose sugar is locked in a C3’-endo conformation by introduction of a 2’-O,4’-C methylene bridge. PMOs contain a backbone of hexagonal morpholine rings linked by phosphorodiamidate bonds.
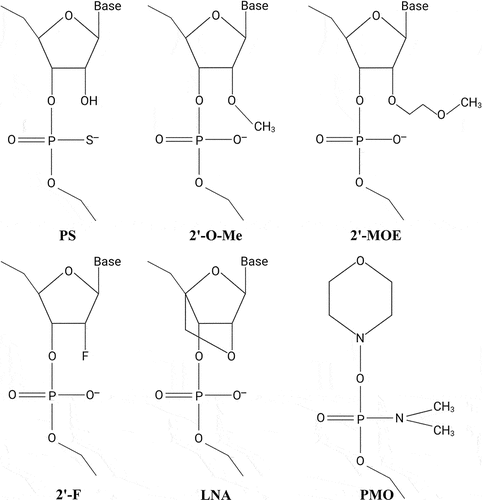
Figure 2. Schematic illustration depicting siRNA and ASO mechanisms of action. A: In the cytoplasm, siRNA triggers are incorporated into the RNA-induced silencing complex (RISC); a ribonucleoprotein complex consisting of Dicer, the RNA binding protein (TRBP), and the RNase Argonaute 2 (AGO2). Upon RISC loading, the strand with the less thermodynamically stable 5’-end is incorporated and guides the RISC to the complementary target mRNA. The mRNA target dissociates from the intact siRNA after AGO2 cleavage, freeing RISC to regenerate and cleave additional mRNA targets. B: ASOs modulate target mRNA expression either by steric blocking (mixmers) or recruitment of RNase H (gapmers). Gapmers are composed of a central DNA region flanked at both ends by chemically modified ribonucleotides and recruit RNase H, which recognizes and cleaves DNA:RNA heteroduplexes.
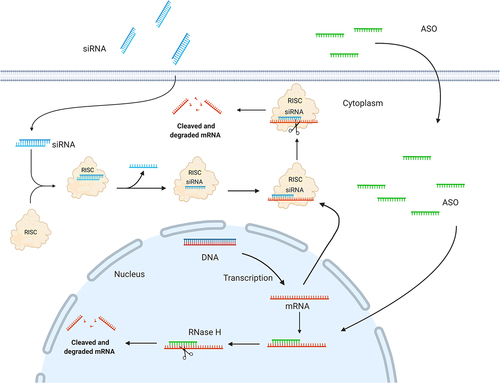
Figure 3. Delivery of RNA therapeutics to the CNS. Schematic illustration depicting the delivery of RNA therapeutics to the CNS. A: Due to the inability to cross the blood-brain barrier, RNA drugs are currently delivered either by intrathecal (IT), intracerebroventricular (ICV), or intraparenchymal injections. B: RNA drugs penetrate and distribute in the brain parenchyma, where they will encounter the different cells of the brain. C: Cellular uptake of RNA drugs can either occur by clathrin- or caveolin-dependent endocytosis, macropinocytosis or other non-productive pathways. CL: Clathrin, CV: Caveolin, EE: Early endosome, LE: Late endosome, LY: lysosome, MA: Macropinocytosis, R: Receptor.
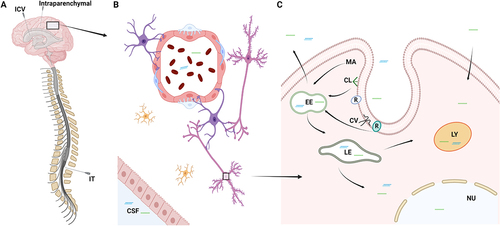
Table 1. RNA therapeutics in clinical development for treatment of neurological and neuromuscular diseases
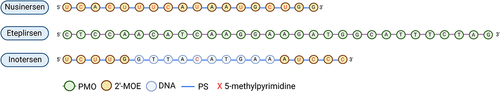
Figure 4. Chemical compositions of nusinersen, eteplirsen, and inotersen. Nusinersen is an 18-nucleotide fully phosphorothioate (PS)-modified 2’-O-methoxyethyl (2’-MOE) ASO targeting survival motor neuron 2 (SMN2) for the treatment of spinal muscular atrophy (SMA). Eteplirsen is a 30-nucleotide PMO-based drug targeting exon 51 of the Dystrophin gene (DMD). Inotersen is a 20-mer gapmer ASO targeting transthyretin (TTR). The ASO is fully PS-modified with five 2ʹ-MOE-modified ribonucleotides at each terminus (i.e. 5–10-5 structure).