Figures & data
Figure 1. RNA modifications with functions of regulators (writers, erasers and readers). m6A, m5C, m7G and m1A methylations in mRNA, tRNA, rRNA and ncRNA are involved in RNA processing and metabolism.
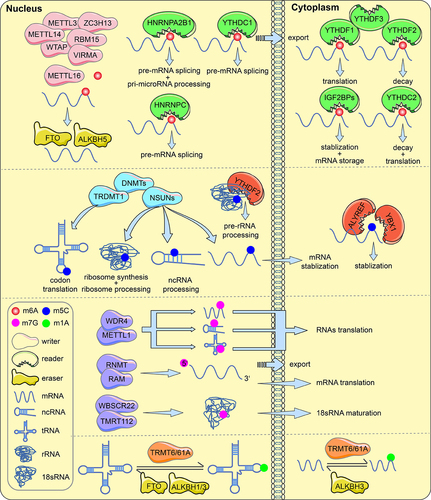
Table 1. The main role of regulators of RNA modification related with prognosis of ovarian cancer.
Figure 2. Diagram of RNA modification regulators important for ovarian cancer growth, invasion, migration, and apoptosis. By hindering the miR-126-5p-targeted suppression of PTEN and hence obstructing the PI3K/Akt/mTOR pathway, METTL3 aids in the development and carcinogenesis of ovarian cancer. The AKT pathway was activated by METTL3, which also encouraged proliferation, invasion, and migration. By focusing on TROAP, METTL14 encouraged procreation, invasion, and migration while hindering procreation capacity. HIF-1‘s upregulation of WTAP expression interferes with miRNA processing and speeds up tumour growth, invasion, and migration. By enlisting RBM15, UBA6-AS1 boosted the m6A methylation of UBA6 mRNA. IGF2BP1 was discovered to be the m6A reader protein of the UBA6-AS1-RBM15-mediated m6A modification of UBA6 mRNA, which improved UBA6 mRNA stability and inhibited the proliferation, migration, and invasion of OC cells functionally. YTHDF1 aided in the carcinogenesis of ovarian cancer by promoting the proliferation, invasion, and migration of cells, as well as increasing the translation of EIF3C in an m6A-dependent manner. MiR-145 inhibited YTHDF2‘s ability to increase the proliferation and migration of EOC cell lines, and FBW7 inhibits YTHDF2-mediated BMF mRNA degradation in ovarian cancer to slow tumour growth and development. Through raising NANOG mRNA expression, ALKBH5 encouraged OC cells to be aggressive and anti-apoptosis. The expression of ALKBH5 was increased, which facilitated the growth and invasion of epithelial ovarian cancer cells. ALKBH5 also activates the mTOR pathway to control autophagy and facilitates the connection between Bcl-2 and Beclin1, which is dependent on m6A. In ovarian cancer, NSUN2 did not significantly control cell migration and proliferation. TRMT10C encouraged OC migration, invasion, and proliferation. In order to encourage the invasiveness of ovarian cancer cells, ALKBH3 improved the stability of CSF-1.
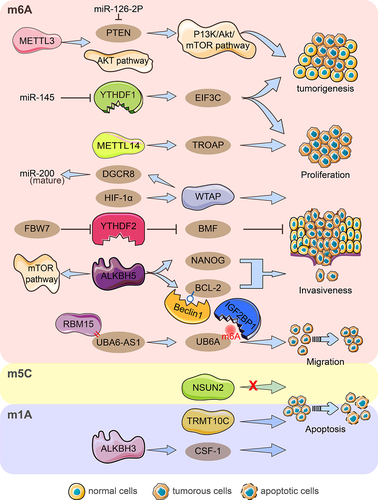
Figure 3. Diagram illustrating the role of RNA modification regulators in ovarian cancer spread, OCSC pathogenesis, TME, and immune infiltration. Metastasis: Through METTL3-mediated m6A alteration of TRPC3 mRNA, PLAA inhibits ovarian cancer metastasis. RBM15 encouraged metastasis of OC, while YTHDF1 inhibited it. CSC: In ovarian cancers and CSC, FTO expression is reduced, and the cAMP pathway which is important in stemness and tumour initiation is controlled. The cisplatin-resistant ovarian cancer cells’ stem cell-like phenotype is made possible by TRIM29 overexpression caused by m6A-YTHDF1. TME: TLR4 upregulates ALKBH5 expression in ovarian cancer cells co-cultured with M2 macrophages via activating the NF-B pathway, eventually driving ovarian carcinogenesis in the TME. Upregulation of WTAP expression by HIF-1α intercedes with miRNA processing, accelerates the Warburg impact. Immune infiltration: In ovarian cancer, YTHDF1, RBM15, ZC3H13, IGF2BP2, and the m1A-B cluster cloud mediate various degrees of immune infiltration.
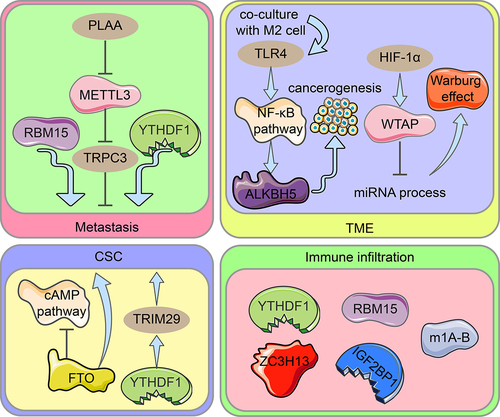
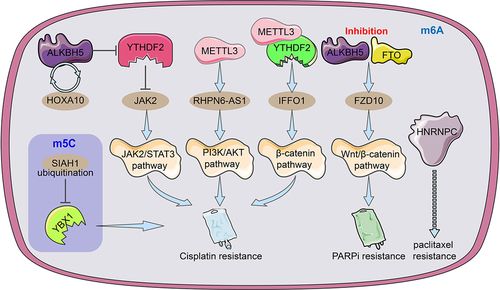
Figure 4. An illustration of the regulators of RNA alteration responsible for ovarian cancer medication resistance. The m6A regulator HNRNPC may be related to and be able to foretell OC resistance to paclitaxel. By facilitating JAK2 m6A demethylation, the ALKBH5-HOXA10 loop promotes EOC resistance to cisplatin by activating the JAK2/STAT3 signalling pathway simultaneously. METTL3-mediated m6A modification of RHPN1-AS1 accelerates cisplatin resistance by activating PI3K/AKT pathway. The METTL3/YTHDF2 axis regulated the mRNA stability of IFFO1 in an m6A-dependent manner and enhances cisplatin resistance. The ubiquitination of YBX-1 caused the target m5C-modified mRnas to become unstable, which made EOC cells more sensitive to the chemotherapy drug cisplatin. To increase FZD10 mRNA m6A modification, activate the Wnt/-catenin pathway, and improve PARPi resistance, it was necessary to downregulate the m6A demethylases FTO and ALKBH5.
Data availability statement
Not available.
