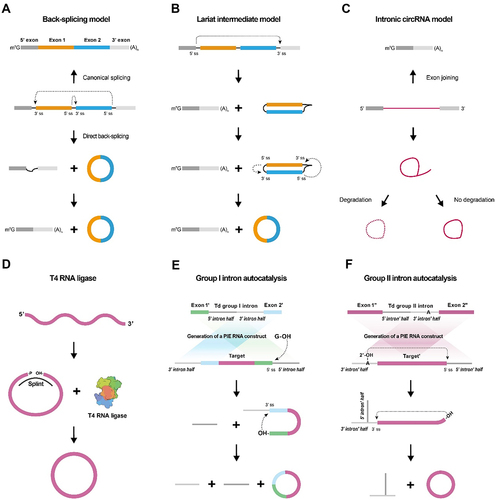
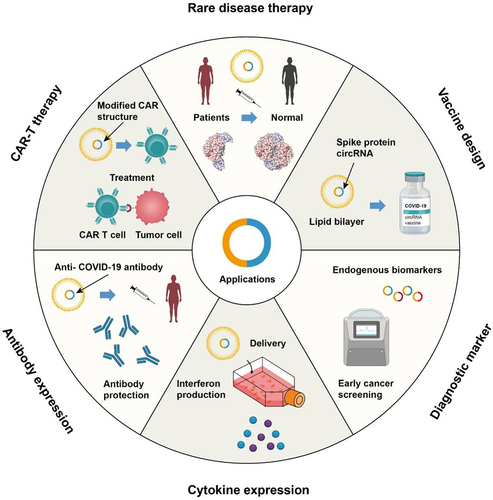
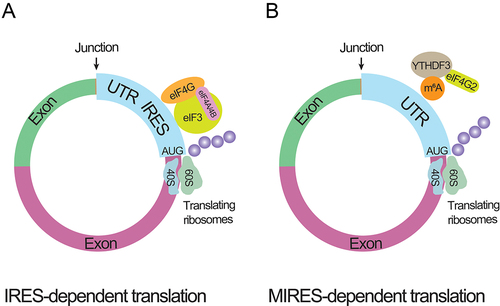
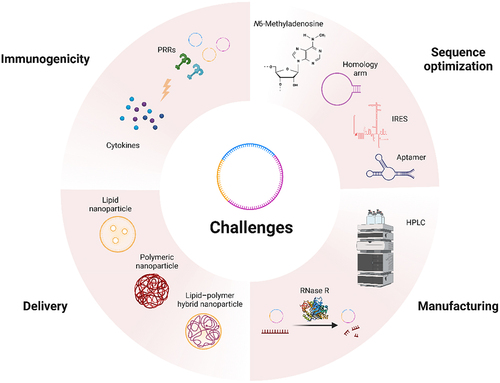
Figures & data
Table 1. The production and function of viral-derived circRNAs.
Table 2. The translational usage of circRNA.
Legnini I, Di Timoteo G, Rossi F, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Molecular Cell. 2017;66(1):22–37.e9. doi: 10.1016/j.molcel.2017.02.017 Paludan SR, Pradeu T, Masters SL, et al. Constitutive immune mechanisms: mediators of host defence and immune regulation. Nat Rev Immunol. 2021;21(3):137–150. doi: 10.1038/s41577-020-0391-5 Pamudurti NR, Bartok O, Jens M, et al. Translation of CircRNAs. Molecular Cell. 2017;66(1):9–21.e7. doi: 10.1016/j.molcel.2017.02.021 Chen X, Yang T, Wang W, et al. Circular RNAs in immune responses and immune diseases. Theranostics. 2019;9(2):588–607. doi: 10.7150/thno.29678 Ungerleider N, Concha M, Lin Z, et al. The Epstein Barr virus circRnaome. PLOS Pathog. 2018;14(8):e1007206. doi: 10.1371/journal.ppat.1007206 Granados-Riveron JT, Aquino-Jarquin G. The complexity of the translation ability of circRnas. Biochim Biophys Acta Gene Regul Mech. 2016;1859(10):1245–1251. doi: 10.1016/j.bbagrm.2016.07.009 Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–120. doi: 10.1038/nature12730 Lu M, Zhang Z, Xue M, et al. N6-methyladenosine modification enables viral RNA to escape recognition by RNA sensor RIG-I. Nat Microbiol. 2020;5(4):584–598. doi: 10.1038/s41564-019-0653-9 Baquero-Perez B, Geers D, Díez J. From a to m6A: the emerging viral epitranscriptome. Viruses. 2021;13(6):1049. doi: 10.3390/v13061049 Tan K, Lim Y. Viruses join the circular RNA world. FEBS J. 2021;288(15):4488–4502. doi: 10.1111/febs.15639 Du L, Wang X, Liu J, et al. A Previously undiscovered circular RNA, circTNFAIP3, and its role in coronavirus replication. MBio. 2021;12(6):e02984–21. doi: 10.1128/mBio.02984-21 Zhang X, Liang Z, Wang C, et al. Viral circular RNAs and their possible roles in virus-host interaction. Front Immunol. 2022;13:939768. doi: 10.3389/fimmu.2022.939768 Role of noncoding RNAs in dengue virushost interaction. Front Biosci (Schol Ed) 2021;13:44. Cai Z, Lu C, He J, et al. Identification and characterization of circRnas encoded by MERS-CoV, SARS-CoV-1 and SARS-CoV-2. Brief Bioinform. 2021;22(2):1297–1308. doi: 10.1093/bib/bbaa334 Chu Q, Zheng W, Su H, et al. A highly conserved circular RNA, circRasgef1b, enhances antiviral immunity by regulating the miR-21-3p/MITA pathway in lower vertebrates. J Virol. 2021;95(7):e02145–20. doi: 10.1128/JVI.02145-20