Figures & data
Figure 1. Representation of domain structures of human Drosophila behavior/human splicing (DBHS) family proteins. Human DBHS family consists of polypyrimidine tract-binding protein-associated splicing factor (PSF)/splicing factor proline/glutamine rich (SFPQ), non-POU domain-containing octamer-binding protein (NONO)/nuclear RNA-binding protein, 54 kDa (p54nrb) and paraspeckle component 1 (PSPC1)/paraspeckle protein 1 (PSP1). DBHS family proteins contain the conserved DBHS region that is involved in RNA recognition and the formation of DBHS family protein homodimer and heterodimer. The N- and C-terminal regions adjacent to the DBHS region are low-complexity regions (LCRs) that are involved in the regulation of phase separation. DBD, DNA-binding domain; LCR, low-complexity region; NOPS, NonA/paraspeckle domain; RRM1, RNA recognition motif 1; RRM2, RNA recognition motif 2.
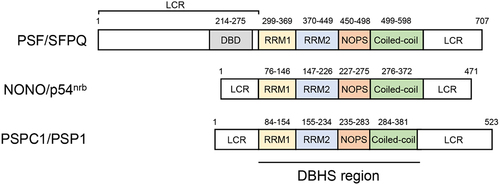
Figure 2. Schematic representation of roles of DBHS family proteins in cells and tissues. (a) polypyrimidine tract-binding protein-associated splicing factor (PSF)/splicing factor proline/glutamine rich (SFPQ) regulates neural gene expression posttranscriptionally and transcriptionally. SFPQ-mediated gene regulation plays important roles in the development and homeostasis of neural tissues and cells and in the pathophysiology of neural diseases. SFPQ is also involved in the development of skeletal muscle and modulation of T cell function. (b) Non-POU domain-containing octamer-binding protein (NONO) posttranscriptionally regulates the rhythmic expression of glucose metabolism-related genes, such as glucokinase (Gck) and glucose transporter type 2, liver (Glut2), and fat metabolism genes, such as ATP-citrate-lyase (Acly) and mitochondrial glycerol-3-phosphate acyltransferase (Gpam), in liver, contributing to the homeostasis of glucose and fat metabolism. In addition, NONO promotes the ten-eleven translocation 1 (TET1)-mediated 5-hydroxymethylcytosine modification of neural genes, facilitating neural differentiation of embryonic stem cells (ESCs). Paraspeckle component 1 (PSPC1) enhances nuclear export of mRNAs associated with adipogenesis to promote adipocyte differentiation. In addition, PSPC1 forms a complex with a 5-methylcytosine (5mC) dioxygenase ten-eleven translocation 2 (TET2) and is involved in transcriptional and posttranscriptional regulation of endogenous retrovirus (ERV), such as murine endogenous retrovirus-L (MERVL) and class II ERVs. NONO and PSPC1 transcriptionally upregulate the expression level of aldehyde dehydrogenase 1 family member a1 (ALDH1A1) to protect Sertoli cells from a toxicant mono-(2-ethylhexyl) phthalate (MEHP). NONO/PSPC1 complex is also suggested to be involved in epigenetic regulation of bivalent genes during the transition from embryonic stem cells (ESCs) to epiblast-like stem cells (EpiLCs). Posttranscriptional and transcriptional target genes of DBHS family proteins are shown in red and green, respectively. Genes regulated by DBHS family proteins during subcellular mRNA transport are shown in blue. Acly, ATP-citrate-lyase; Acsl1, acyl-coenzyme a synthetase long chain family member 1; ALDH1A1, aldehyde dehydrogenase 1 family member a1; APP, amyloid-β precursor protein; Bclw, B-cell lymphoma-2 (bcl-2)-like 2; CaMKIIα, calmodulin-dependent protein kinase II α; CD45, cluster of differentiation 45; DBHS, Drosophila behavior and human splicing; Ebf1, early B-cell factor 1; EpiLCs, epiblast-like stem cells; ERV, endogenous retrovirus; ESCs, embryonic stem cells; Gck, glucokinase; Glut2, glucose transporter type 2, liver; Gpam, mitochondrial glycerol-3-phosphate acyltransferase; KIF5, kinesin family member 5; Lmnb2, lamin B2; MAPT, microtubule associated protein tau; MEHP, mono-(2-ethylhexyl) phthalate; MERVL, murine endogenous retrovirus-L; NMHC II-B, nonmuscle myosin heavy chain II-B; NONO, non-POU domain-containing octamer-binding protein; Pparg, peroxisome proliferator activated receptor γ; PSPC1, paraspeckle component 1; Scd1, stearoyl-coenzyme A desaturase-1; SFPQ, splicing factor proline/glutamine rich; 3R-T, 3-repeat tau; 4R-T, 4-repeat tau; TET1, ten-eleven translocation 1; TET2, ten-eleven translocation 2.
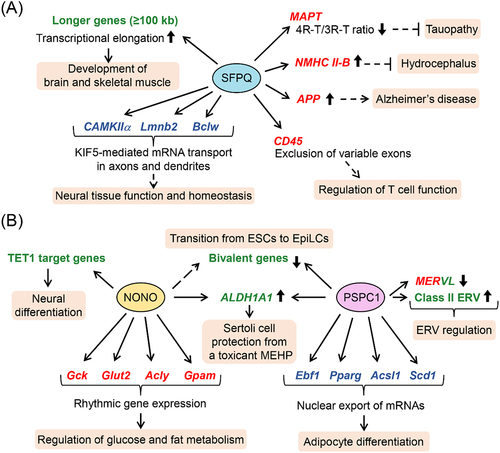
Table 1. Roles of DBHS family proteins in gene regulation in cells and tissues.
Table 2. Roles of DBHS family proteins in gene regulation in cancers.
Figure 3. Diagram of gene regulation by DBHS family proteins in cancers. Posttranscriptional and transcriptional target genes of DBHS family proteins are shown in red and green, respectively. Genes regulated by DBHS family proteins during the internal ribosome entry site (IRES)-mediated translation are shown in orange. AR, androgen receptor; ASPM, assembly factor for spindle microtubules; BIN1, bridging integrator 1; CASP9, caspase 9; CK1α, casein kinase 1 α; CRPC, castration-resistant prostate cancer; CTBP1, C-terminal binding protein 1; CXCL8, C-X-C motif chemokine ligand 8; E2F8, E2F transcription factor 8; ER, oestrogen receptor; ESR1, estrogen receptor 1; Ets-1, v-ets erythroblastosis virus E26 oncogene homolog 1; FOSL1, FOS like antigen 1; GATA2, GATA-binding protein 2; GPX1, glutathione peroxidase 1; HAND2, heart and neural crest derivatives expressed 2; HIF-1/2, hypoxia-inducible factor-1/2; LDHA, lactate dehydrogenase A; MycN, neuroblastoma MYC oncogene; NONO, non-POU domain-containing octamer-binding protein; Oct4, octamer-binding protein 4; PGK1, phosphoglycerate kinase 1; PSPC1, paraspeckle component 1; RUNX2, runt-related transcription factor 2; SCFD2, sec1 family domain-containing 2; SETMAR, SET domain and mariner transposase fusion protein; SFPQ, splicing factor proline/glutamine rich; SKP2, S-phase-associated kinase 2; SLC7A11, solute carrier family 7 member 11; SMAD3, Sma- and Mad-related protein 3; Sox2, SRY-box transcription factor 2; STAT3, signal transducer and activator of transcription 3; TAZ, transcription factor PDZ-binding motif; TGFB1, transforming growth factor-β1; TNBC, triple-negative breast cancer; VEGFA, vascular endothelial growth factor A.
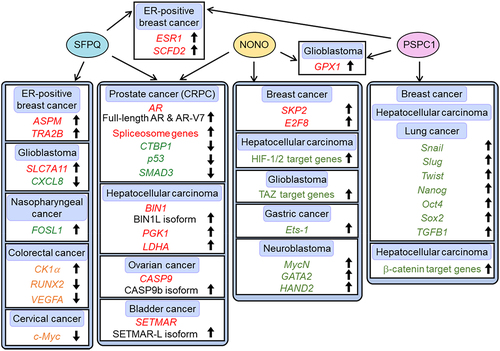
Table 3. Small molecules targeting DBHS family proteins and their tumour-suppressive effects.
