Figures & data
FIG. 1 Schematic structure of Type I and Type II cytokine receptors. Ig-like domain: immunoglobulin-like domain; Cys: cysteine; WS motif: WSXWS sequence (W: tryptophane; S: serine; X: non-conserved amino acid).
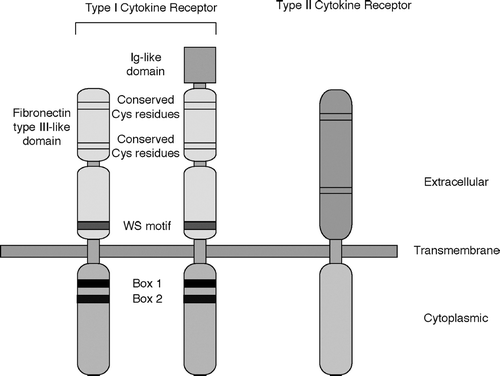
FIG. 2 Schematic structure of Jak kinases (A) and Stat proteins (B). Jak kinases have seven regions with sequence similarity. JH: Jak homology. Stat proteins have six domains with sequence similarity. SH2: Src homology 2 domain.
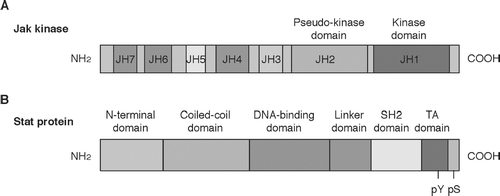
FIG. 3 The Jak-Stat pathway. Binding of cytokines to their specific receptors leads to dimerization or oligomerization of cytokine receptor subunits, making receptor-associated Jak kinases close to each other. This induces cross-phosphorylation of Jak kinases, leading to their maximal activation. Activated Jak kinases in turn phosphorylate tyrosine residues of the cytoplamic domains of cytokine receptors. These phosphorylated tyrosines are recognized by the SH2 domains of Stat proteins, and Stat proteins bind to the cytoplasmic domains of the cytokine receptors. Then, receptor-bound Stat proteins are phosphorylated by Jak kinases at their carboxyl-termini. Phosphorylated Stat proteins are released from the cytoplasmic domains of cytokine receptors and form homodimers, heterodimers, or heterotrimers via the interaction of their SH2 domains and phosphorylated tyrosines. These complexes are transported into the nucleus by importins. The Stat complexes bind to their specific recognition sequences of their target genes and regulate their expression.
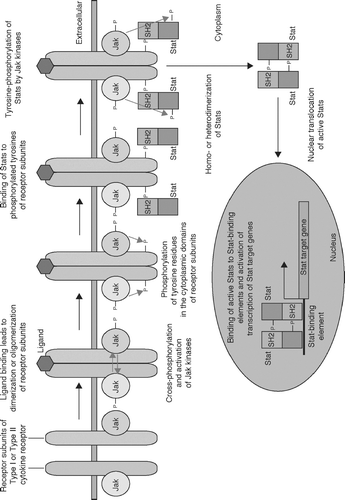
TABLE 1 Negative regulators of Stat proteins