Figures & data
Figure 1. Effect of AF-SWCNT on generation of NK cell responses in vitro and in vivo. NK cells were activated in vitro by culturing spleen cells with IL-2/ml in absence or presence of AF-SWCNT/ml as described in the “Materials and methods” section. Cells were washed and their cytotoxic activity against YAC target cells were determined at E:T ratios of 100, 50, 25 and 12.5 in a 4-h chromium release assay. Values shown from a representative experiment are means [±SEM] of percent target lysis at different E:T ratios for NK cells activated with IL-2 in the absence (O = 0 μg/ml) or presence (▾ =10 μg, Δ = 25 μg, ▪ = 50 μg/ml) of AF-SWCNT [main Panel A]. Lytic units of cytotoxic activity were calculated for NK cells activated with IL-2 in presence of various concentrations of AF-SWCNT. Inset figure in Panel A shows dose-related decline in lytic units of NK cell activity generated in presence of different concentrations of AF-SWCNT. *p < 0.05 vs. AF-SWCNT untreated (ANOVA). Similar results for in vivo activation of NK cells by Poly(I:C) are shown in Panel B. Different groups of C57BL/6 mice were administered Poly(I:C) along with AF-SWCNT as described in “Materials and methods” section. In main Panel B, % target lysis at different E:T ratios of cells from Poly(I:C)-treated mice injected without (O = 0 μg/mouse) or with (▾ =10 μg, Δ = 50 μg, ▪ = 100 μg/mouse) AF-SWCNT are shown. Inset figure in Panel B reflects a dose-related inhibition of NK activation by AF-SWCNT. *p < 0.05, **p < 0.01 vs. AF-SWCNT untreated (ANOVA).
![Figure 1. Effect of AF-SWCNT on generation of NK cell responses in vitro and in vivo. NK cells were activated in vitro by culturing spleen cells with IL-2/ml in absence or presence of AF-SWCNT/ml as described in the “Materials and methods” section. Cells were washed and their cytotoxic activity against YAC target cells were determined at E:T ratios of 100, 50, 25 and 12.5 in a 4-h chromium release assay. Values shown from a representative experiment are means [±SEM] of percent target lysis at different E:T ratios for NK cells activated with IL-2 in the absence (O = 0 μg/ml) or presence (▾ =10 μg, Δ = 25 μg, ▪ = 50 μg/ml) of AF-SWCNT [main Panel A]. Lytic units of cytotoxic activity were calculated for NK cells activated with IL-2 in presence of various concentrations of AF-SWCNT. Inset figure in Panel A shows dose-related decline in lytic units of NK cell activity generated in presence of different concentrations of AF-SWCNT. *p < 0.05 vs. AF-SWCNT untreated (ANOVA). Similar results for in vivo activation of NK cells by Poly(I:C) are shown in Panel B. Different groups of C57BL/6 mice were administered Poly(I:C) along with AF-SWCNT as described in “Materials and methods” section. In main Panel B, % target lysis at different E:T ratios of cells from Poly(I:C)-treated mice injected without (O = 0 μg/mouse) or with (▾ =10 μg, Δ = 50 μg, ▪ = 100 μg/mouse) AF-SWCNT are shown. Inset figure in Panel B reflects a dose-related inhibition of NK activation by AF-SWCNT. *p < 0.05, **p < 0.01 vs. AF-SWCNT untreated (ANOVA).](/cms/asset/9fc436db-9e78-4adb-bd48-f18cdd59a15c/iimt_a_1191562_f0001_b.jpg)
Table 1. Suppression of NK cell generation and activation in response to IL-2 (in vitro) and Poly(I:C) (in vivo) by AF-SWCNT.
Figure 2. Effect of AF-SWCNT on IL-2-activated NK cell sub-populations in vitro. Mouse spleen cells (2 × 106/ml) were cultured with 500 U IL-2/ml in absence (□) or presence (▪) of 50 μg AF-SWCNT/ml. After 72 h, cells were washed and double-stained with a combination of antibodies against NK1.1 and CD107a markers or NK1.1 and FasL markers. Values shown are means ± SEM from four independent experiments. **p < 0.01 vs. AF-SWCNT untreated (Student’s t-test).
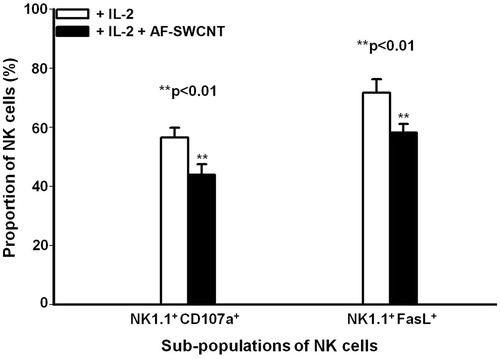
Table 2. Intracellular expression of IFNγ and TNFα by NK cells derived from mice treated with poly I:C with or without AF-SWCNT.
Figure 3. Uptake of FAF-SWCNT by control and IL-2-induced NK1.1 cells in culture. Mouse spleen cells (2 × 106/ml) were cultured in the absence (control) or presence of 500 U IL-2/ml along with 2 μg FAF-SWCNT/ml. After 12, 24, 48 or 72 h, cells were washed, stained with NK1.1 mAb and analyzed on a flow cytometer using NK 1.1 and FAF-SWCNTs [colors on X and Y axis, respectively]. Representative histograms for control and IL-2-activated cells at 24 h time-point are shown in Panels A and B, respectively. Values shown in each quadrangle indicate are the means [±SEM] fractions of cells in that quadrangle, from three replicate experiments. At all four timepoints, the percentages of NK 1.1+FAF-SWCNT+ cells as a percentage of all NK1.1+ cells were computed from flow histograms and plotted vs. time (Panel C). Values shown are mean [± SEM] percentages of NK cells positive for FAF-SWCNT at all timepoints, from three replicate experiments (Panel C). *p < 0.05, **p < 0.01 vs. control (Student’s t-test).
![Figure 3. Uptake of FAF-SWCNT by control and IL-2-induced NK1.1 cells in culture. Mouse spleen cells (2 × 106/ml) were cultured in the absence (control) or presence of 500 U IL-2/ml along with 2 μg FAF-SWCNT/ml. After 12, 24, 48 or 72 h, cells were washed, stained with NK1.1 mAb and analyzed on a flow cytometer using NK 1.1 and FAF-SWCNTs [colors on X and Y axis, respectively]. Representative histograms for control and IL-2-activated cells at 24 h time-point are shown in Panels A and B, respectively. Values shown in each quadrangle indicate are the means [±SEM] fractions of cells in that quadrangle, from three replicate experiments. At all four timepoints, the percentages of NK 1.1+FAF-SWCNT+ cells as a percentage of all NK1.1+ cells were computed from flow histograms and plotted vs. time (Panel C). Values shown are mean [± SEM] percentages of NK cells positive for FAF-SWCNT at all timepoints, from three replicate experiments (Panel C). *p < 0.05, **p < 0.01 vs. control (Student’s t-test).](/cms/asset/00363dbc-2aae-4599-986d-24d9cfefc3d5/iimt_a_1191562_f0003_b.jpg)
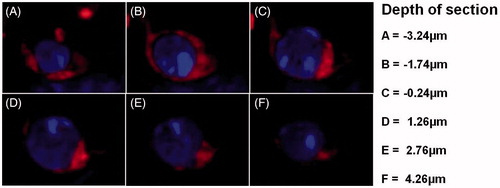
Figure 4. Intracellular distribution of FAF-SWCNT internalized by IL-2-activated NK1.1+ cells. Mouse spleen cells (2 × 106/ml) were cultured in presence of 500 U IL-2/ml and 2μg FAF-SWCNT/ml. After 24 h, cells were washed, stained with anti-mouse NK1.1 mAb, and NK1.1+ cells were sorted on a fluorescence activated cell sorter. Purified activated NK cells were then stained with DAPI and examined under a confocal microscope. Representative confocal images show combined DAPI/FAF-SWCNT fluorescent image at different depths (Z: -3.24 to 4.26 μm) (Magnification =60×).