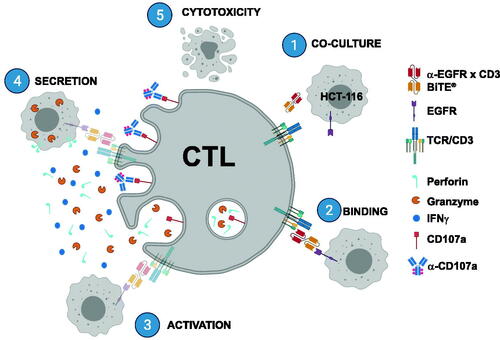
Figures & data
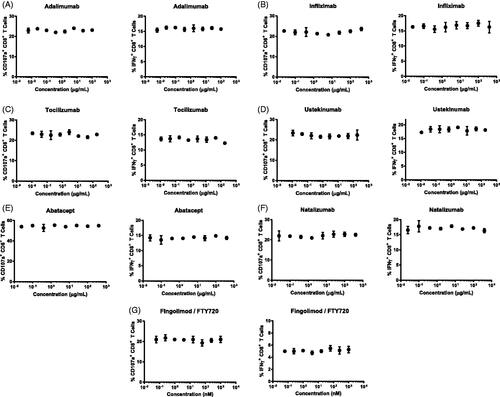
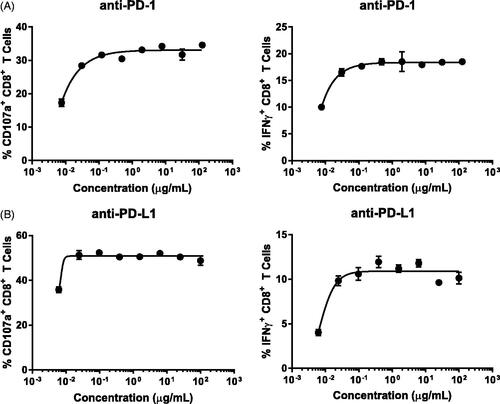
Table 1. Immunomodulatory agents and mechanisms of action.
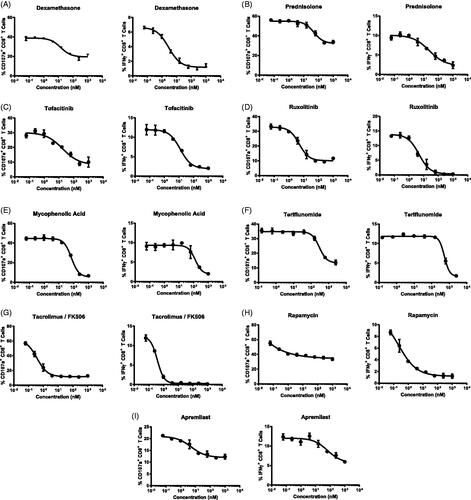
Table 2. IC50 values of immunosuppressant agents.