Figures & data
Table 1. Participant characteristics.
Figure 1. Standardized treatment effect estimates for preliminary effects. Effect estimates for average soreness/discomfort, average stiffness, daily activity interference, physical activity interference, worst soreness/discomfort, worst stiffness, muscle circumference, and relaxed elbow angle were reflected so that all positive effect estimates indicate active superior to placebo and all negative effect estimates indicate placebo superior to active. The dotted vertical blue line indicates minimally clinical important difference of 0.40; the dotted vertical black line indicates null effect.
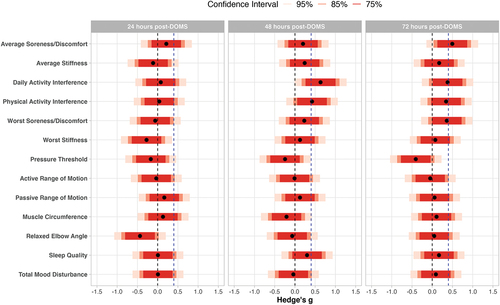
Table 2. Unstandardized and standardized mean difference of active study formulation from placebo and standard errors.
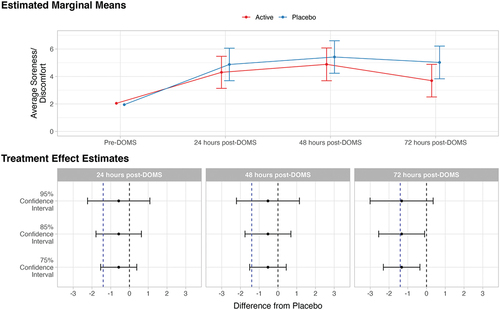
Figure 2. Estimated marginal means and treatment effect estimates for the effect of average soreness or discomfort. In the estimated marginal means panel, bars represent 95% confidence intervals. In the treatment effect estimates panel, the dotted vertical blue line indicates minimally clinical important difference of 1.40 (lv et al., 2020; Tashjian et al., 2009); the dotted vertical black line indicates null effect.