Figures & data
Figure 1. Study design: Visit schedule.
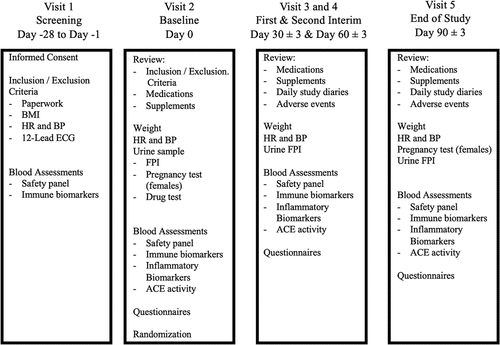
Table 1. Exclusion criteria.
Table 2. Immune system, safety endpoint, and Foundational Pain Index biomarkers.
Figure 2. Consort diagram.
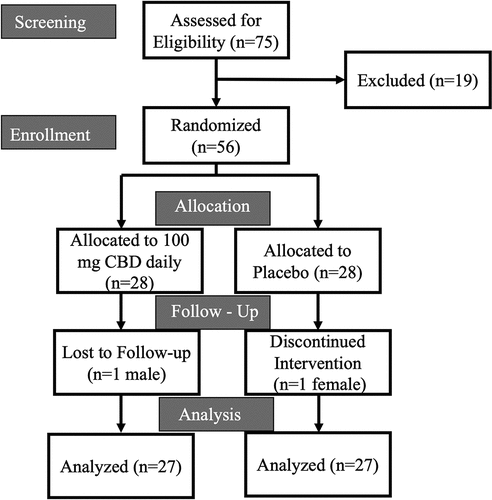
Table 3. Baseline participant descriptive data.
Table 4. Changes in blood biomarkers by group and sex.
Table 5. Changes in immune system biomarkers by group and sex.
Table 6. Changes in perceived stress, sleep quality, and body discomfort by group and sex.
Table 7. Changes in profile of mood states total mood disturbance and subscales by group and sex.
Table 8. Changes in missed productivity by group and sex.
Table 9. Changes in subscales of foundational pain index by group and sex.
