Figures & data
Figure 1. USP30 and USP35 are mitochondrial DUBs that may affect mitophagy. (A-C) HeLa cells expressing USP30-GFP (A), USP35-GFP (B), or USP20-GFP (C) were fixed and immunostained for GFP 24 h after transfection. anti-ATP5A1 antibody was used to identify mitochondria. (D-I) HeLa cells stably expressing GFP-PARK2 were transfected with siRNA against USP30 for 72 h. Cells were treated with 10 µM CCCP for 0, 1, 3, and 6 h as indicated and the cell lysates were examined for MFN2, VDAC1, and ubiquitinated-TOMM20 levels by immunoblotting. The average of normalized density of MFN2 and monoubiquitinated TOMM20 was plotted (n = 3, P < 0.05). (F-I) The same experiment was performed with cells that received siRNA against USP35 (F) or USP20 (H). The average of normalized density of MFN2 and monoubiquitinated TOMM20 was also analyzed (G-I). Scale bar: 10 µm.
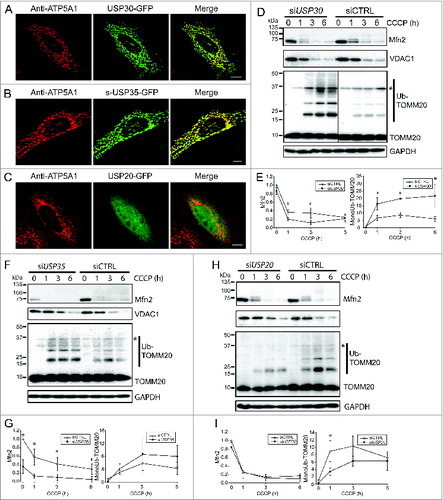
Figure 2. Detailed analysis of USP30 and USP35 localization. (A) HeLa cells transfected with USP30-HA for 16 h were treated with either CCCP or DMSO for 2 h. These cells were fixed and immunostained with anti-TOMM20 (red) and anti-HA antibodies (green). (B) s-USP35-HA was expressed in HeLa cells for 16 h. Cells were then treated with DMSO or CCCP for 2 h. In order to recover mitochondrial potential, cells treated with CCCP for 2 h were washed once with 1× DPBS and were incubated in regular growth media for 2 h. Cells were then fixed and immunostained with anti-HA antibody. (C) HeLa cells expressing s-USP35 were treated with DMSO, CCCP, or allowed for potential recovery for 2 h. Mitochondrial fractions (mito) were isolated from the cytosolic fraction (cyto). Total cell lysates (TCF), cyto, and mito fractions were examined by SDS-PAGE and immunoblotting. (D) FLAG-l-USP35 was cotransfected with mitochondrial-targeting RFP (mtRFP) for 16 h in HeLa cells. Cells were fixed and immunostained with anti-FLAG antibody. (E) HeLa cells were cotransfected with FLAG-l-USP35 and s-USP35-HA and then treated with CCCP or DMSO for 2 h as indicated. Before fixation, cells were treated with digitonin to remove the cytosolic signal of l-USP35. Fixed cells were immunostained with anti-FLAG and anti-HA antibodies. (F) FLAG-l-USP35 and s-USP35-HA were transfected individually or cotransfected into HeLa cells for 24 h. Cells were then treated with CCCP or DMSO for 2 h. Cleared cell lysates were immunoprecipitated for FLAG tagged proteins using anti-FLAG antibody and protein A-agarose beads. Protein contents were analyzed on SDS-PAGE and immunoblot with anti-FLAG and anti-HA antibodies. Scale bar: 10 µm.
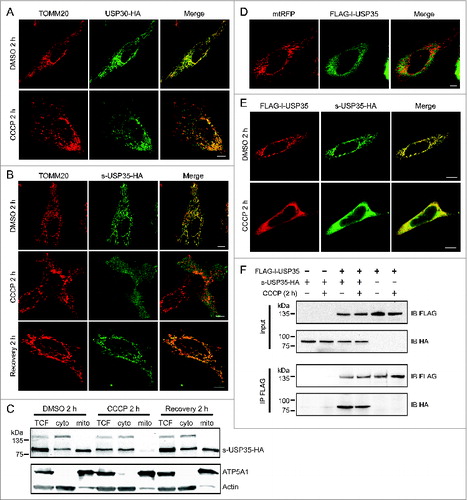
Figure 3. USP30 and USP35 delay PARK2-mediated mitophagy. (A) Schematic representation of the mCherry-GFP-lysosome assay. The ORF of mCherry and GFP in tandem is tagged with an outer mitochondrial membrane-targeting transmembrane domain (RG-OMMTM). When localized in the cytosol and autophagosomes, mCherry and GFP are both fluorescent, resulting in yellow mitochondria. In a low pH environment such as the lysosomes, only the signal of GFP is quenched, resulting in red mitochondria. (B-D) HeLa cells treated with nontargeting siRNA (siCTRL) (B), siRNA against USP30 (siUSP30) (C) or siRNA against USP35 (siUSP35) (D) were cotransfected with RG-OMMTM and Cer-PARK2 (not shown) for 16 h. Cells were treated with CCCP, E-64, and leupeptin, and were imaged live at 0 h and 6 h of the treatment. (E) Quantification of mCherry-GFP-lysosome assay for siUSP30 and siUSP35 versus siCTRL. The total area of mitochondria and the area of red mitochondria in each cell was measured with imaging software Volocity®6.3. The percentages of mitochondria in lysosome of cells from each treatment were plotted in box plots, and rank-sum significance test was performed. (n = 66 per treatment * P < 0.05). Scale bar: 10 µm.
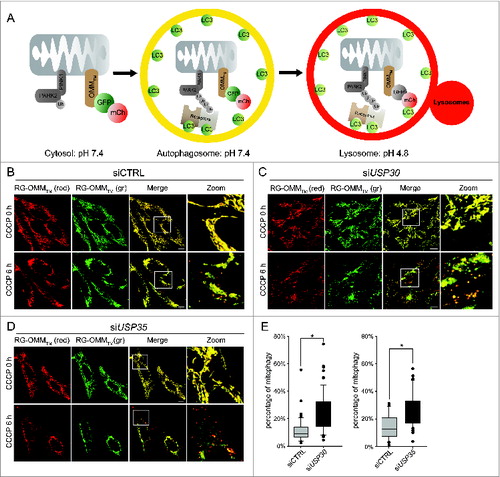
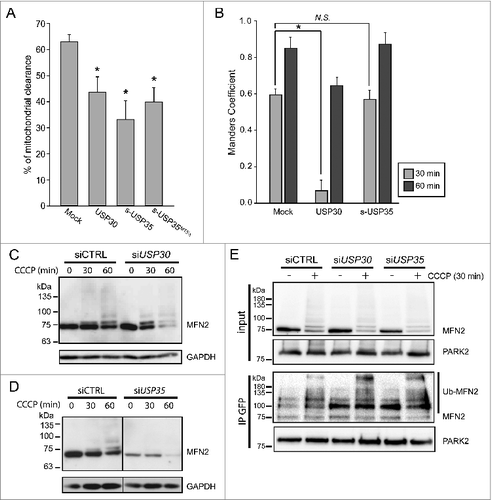
Figure 4. USP30, but not USP35 delays PARK2 recruitment to the mitochondria. (A) GPRM cells were transfected with USP30-HA, s-USP35-HA, or s-USP35MTSΔ-HA for at least 16 h. Cells were treated with 10 µM CCCP for 24 h prior to fixing and staining with an anti-HA antibody. The percentage of the cells with cleared mitochondria was calculated for each treatment (n = 3, P < 0.05). (B) GPRM cells were transfected with USP30-HA, or s-USP35-HA for 16 h. Cells were then treated with 10 µM CCCP and fixed after 30 min or 60 min. Cells were treated with digitonin for 2 min prior to fixation in order to remove nontargeted GFP-PARK2. To visualize USP30, cells were immunostained with anti-HA antibody. Because USP35 is localized to the cytosol during CCCP treatment, USP35 is not detectable in the cells treated with digitonin, however, the transfection efficiency was 75 ± 15% (Fig. S4). Manders coefficient was measured with Volocity®6.3. The average of Manders coefficient was shown (n = 3, * P < 0.05). (C and D) GPRM cells were transfected with siRNA against USP30 (siUSP30) and siRNA against USP35 complex (siUSP35) for 72 h. Nontargeting siRNA (siCTRL) was transfected as control. Cells were treated with 10 µM CCCP for 30 and 60 min. Protein contents were analyzed by immunoblotting with anti-MFN2 antibody. (E) GPRM cells were transfected with control siRNA, siRNA against USP30 and siRNA against USP35 for 72 h. Cells were treated with 10 µM CCCP or DMSO for 30 min. PARK2 was immunoprecipitated from the cell lysates with anti-GFP antibody and Protein A-sepharose beads. The content of the immunoprecipitation was analyzed by SDS-PAGE and immunoblotting with anti-PARK2 and anti-MFN2 antibodies.